2. Xi′ an Modern Chemistry Research Institute, Xi′ an 710065, China
2. 西安近代化学研究所,陕西 西安 710065
Compounds containing nitroxyl group (N—NO2) are of interest for practical use as energetic materials owing to their high energy density and nitrogen content [1-4]. Nitroguanidine (NG) is a simple nitroxyl compound. The effect of electron withdrawing of ni- tro group makes the lower electron density in the single carbon atom, so NG can behave as an active starting material in the reactions with nucleophilic reagents to get a series of NG-based energetic materials [5-9]. Because of the excellent property and low cost, NG has become an important moiety to design and synthesize other new high - nitrogen energetic materials [6-8, 10-12].
Bis (nitroguanidine) methane (BNGM) was report- ed firstly by Yu et al [13]through the reaction of NG and formaldehyde. BNGM was considered as an important intermediate to synthesize other heterocyclic compounds because of the existence of 

In this paper, we mainly report the thermal behavior, specific heat capacity and adiabatic time-to- explosion of BNGM, further estimating its thermal stability and exploring essential application values as an energetic material. The difference of thermal behavior between the parent material and BNGM is also discussed.
2 Experimental 2.1 SynthesisThe parent materials were purchased from the trade, and formaldehyde was AR grade product. The purity of nitroguanidine was 98% and the concentration of hydrochloric acid was 38%. BNGM was prepared according to Ref [15] and the synthetic route of BNGM was showed in Scheme 1. 1H NMR (DMSO - d6, 500 MHz):8.157, 4.597, 3.345. 13C NMR (DMSO-d6, 500 MHz):159.532, 73.329. IR (KBr, ν/cm-1):3319, 3180, 1586, 1542, 1367, 1252, 983, 719. Anal. calcd for C3H8N8O4 (%):C 16.36, H 3.64, N 50.91;Found:C 16.45, H 3.74, N 51.17.
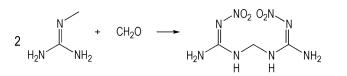
|
Scheme1 Synthetic route of BNGM |
The differential scanning calorimetry (DSC) experiments were performed using a DSC200 F3 (NETZSCH, Germany) apparatus under a nitrogen atmosphere with a flow rate of 80 mL · min-1. The heating rates were 5.0, 7.5, 10.0, 12.5 ℃ · min-1 and 15.0 ℃ · min-1 from ambient temperature to 350 ℃, respectively. The thermogravimetry/differential thermogravimetry (TG/DTG) experiment was determined using a SDT-Q600 apparatus (TA, USA) under a nitrogen atmosphere at the flow rate of 100 mL·min-1. The heating rate used was 10.0 ℃·min-1 from ambient temperature to 500 ℃. The specific heat capacity was determined using a Micro-DSCⅢ apparatus (SETARAM, France), and the sample mass was 196.9 mg. The heating rate used was 0.15 ℃ ·min-1 from 10 ℃ to 80 ℃. Energy of combustion was determined by IKA C5000 oxygenbomb calorimeter (German) adiabatically. The calorimeter was calibrated with the standard substance benzoic acid having a purity of 99.99%, and the sample was tested with 6 times. The impact sensitivity was determined by using a ZBL-B impact sensitivity instrument (Nachcn, China). The mass of drop hammer was 2.0 kg, and the sample mass for test was 30 mg.
3 Results and Discussion 3.1 Thermal Decomposition BehaviorTypical DSC and TG-DTG curves (Fig. 1) indicate that the thermal behavior of BNGM can be divided into two exothermal decomposition processes. The onset temperature, peak temperature and decomposition enthalpy at a heating rate of 10 ℃·min-1 are 205.7 ℃, 208.1 ℃ and 303.3 J·g-1 with a mass loss of about 32.2% for the first decomposition process, and 276.4 ℃, 292.5 ℃, 172.0 J · g-1 with a mass loss of about 31.7% for the second process, respectively. The final residue is about 25.4% at 400 ℃. The DSC curve of NG was also obtained, and there is an endothermic peak as a melting peak at 249.2 ℃. Subsequently, an intense exothermic process occurred at 254.5 ℃ for NG. It can be seen that the DSC curves of NG and BNGM are obviously different. Although BNGM possesses a good thermodynamic stability, it is lower than that of NG and the reason should be the new unstable —NH—CH2—NH— bond of BNGM molecule.

|
Fig.1 Typical DSC curves of BNGM and NG and TG/DTG curves of BNGM at a heating rate of 10 ℃·min-1 |
In order to obtain the kinetic parameters (the apparent activation energy (E) and preexponential constant (A)) of the first exothermic decomposition process, a multiple heating method (Kissinger′s method [17] and Ozawa′s method [18]) was employed according to the data in Fig. 2. The determined values of the beginning temperature (T0), extrapolated onset temperature (Te) and peak temperature (Tp) at different heating rates are listed in Table 1. The values of T00 and Te0 corresponding to heating rate β →0 obtained by Eq. (1) are 164.1 ℃ and 189.6 ℃[19].
| $ {T_{\left ( {{\rm{ 0}}\;\;{\rm{or}}\;\;{\rm{ e }}} \right) i}} = {T_{\left ( {{\rm{ }}00\;\;{\rm{ or}}\;\;{\rm{ e0 }}} \right) }} + n{\beta _{\rm{i}}} + m\beta _i^2 + p\beta _i^3,\;\;i = {\rm{ }}1{\rm{ }} - {\rm{ }}5 $ | (1) |
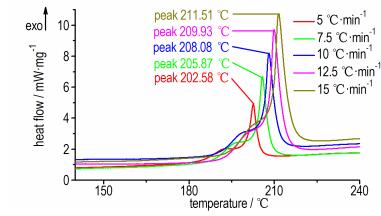
|
Fig.2 DSC curves of BNGM at different heat rates |
| Tab.1 The values of T0, Te, Tp and kinetic parameters of the first decomposition process for BNGM |
where n, m and p are coefficients.
The calculated values of kinetic parameters (E and A) are listed in Table 1. E obtained by Kissinger′s method is consistent with that by Ozawa ′s method. The linear correlation coefficients (r) are all close to 1. So the results are credible.
The self-accelerating decomposition temperature (TSADT) and critical temperature of thermal explosion (Tb) are two important parameters required in evaluation of their safe storage and process operations for energetic materials and then to evaluate the thermal stability. TSADT and Tb for BNGM are calculated as 189.6 ℃ and 190.9 ℃, respectively [19-20], indicat- ing that the thermal stability of BNGM is good.
3.2 Specific Heat CapacityFigure 3 shows the determination result of specific heat capacity (cp) for BNGM, using a continuous specific heat capacity mode of apparatus. It can be seen that cp presents a good linear relationship with temperature in determined temperature range. Specific heat capacity equation is shown as:
| $ {c_p} = 0.1763 + 3.2461 \times {10^{ - 3}}T\left ( {285.0{\rm{ K}} < T < 350.0{\rm{ K}}} \right) $ | (2) |
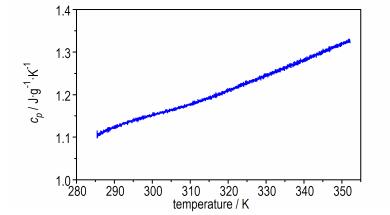
|
Fig.3 Determination results of the continuous specific heat capacity for BNGM |
The specific heat capacity and molar heat capacity of BNGM are 1.15 J·g-1·K-1 and 251.9 J·mol-1·K-1 at 298.15 K, respectively.
3.3 Adiabatic Time-to-explosionThe adiabatic time - to - explosion is also an important parameter for evaluating the thermal stability of energetic materials and can be calculated by Eqs. (3) and (4) [19, 21-25].
| $ {c_p}\frac{{{\rm{d}}T}}{{{\rm{d}}t}} = QA{\rm{exp}}\left ( { - E/RT} \right) f (\alpha ) $ | (3) |
| $ \alpha = \int_{{T_0}}^T {\frac{{{c_p}}}{Q}{\rm{d}}T} $ | (4) |
where cp is the specific heat capacity, J·mol-1·K-1; T is the absolute temperature, K;Q is the exothermic values, J·mol-1; A is the pre-exponential factor, s-1; E is the apparent activation energy of the thermal decomposition reaction, J·mol-1; R is the gas con- stant, J·mol-1·K-1; f (α) is the most probable kinetic model function and α is the conversion degree.
After integrating of Eq. (3), the adiabatic time- to-explosion equation can be obtained as:
| $ t = \int_0^t {{\rm{d}}t} = \int_{{T_0}}^T {\frac{{{c_p}{\rm{exp}}\left ( {E/RT} \right) }}{{QAf (\alpha ) }}{\rm{d}}T} $ | (5) |
where the limit of temperature integration is from T00 to Tb.
In fact, the conversion degree (α) of energetic materials from the beginning thermal decomposition to thermal explosion in the adiabatic conditions is very small, and it is very difficult to obtain the most probable kinetic model function f (α) at the process. So, we separately used Power-low model, Reaction-order model, Avrami- Erofeev model and the above obtained kinetic model function to estimate the adiabatic time - to - explosion and supposed different rate orders (n) [19, 26]. The calculation results are listed in Table 2.
| Tab.2 The calculation results of adiabatic time-to-explosion |
From Table 2, we can see that the calculation results exhibit some deviations and the decomposition model has big influence on the estimate of adiabatic time-to-explosion. From the whole results, we believe the adiabatic time - to - explosion of BNGM should be about 280 s. It is a relatively long time, and the result indicates that BNGM has a good thermal stability.
3.4 Enthalpy of CombustionThe determination results of the energy of constant volume combustion are shown in Table 3, so the constant-volume combustion enthalpy for BNGM is (-10171.4±27.5) J·g-1.
| Tab.3 Determination results of enthalpy of combustion at 298.15 K |
The standard molar enthalpy of combustion (
| $ \begin{array}{l} {{\rm{C}}_{\rm{3}}}{{\rm{H}}_{\rm{8}}}{{\rm{N}}_{\rm{8}}}{{\rm{O}}_{\rm{4}}}\left ( {{\rm{ s }}} \right) {\rm{ }} + {\rm{ }}5{{\rm{O}}_2}\left ( {{\rm{ g }}} \right) {\rm{ }} = {\rm{ }}3{\rm{C}}{{\rm{O}}_{\rm{2}}}\left ( {{\rm{ g }}} \right) {\rm{ }} + {\rm{ }}4{{\rm{H}}_{\rm{2}}}O (l) {\rm{ }} + \\ 3{{\rm{N}}_{\rm{2}}}\left ( {{\rm{ g }}} \right) {\rm{ }} + {\rm{ }}2{\rm{N}}{{\rm{O}}_{\rm{2}}}\left ( {{\rm{ g }}} \right) \end{array} $ | (a) |
| $ 2{\rm{N}}{{\rm{O}}_{\rm{2}}}\left ( {{\rm{ g }}} \right) {\rm{ }} + \frac{2}{3}{{\rm{H}}_{\rm{2}}}{\rm{O}} (l) {\rm{ }} = \frac{4}{3}{\rm{HN}}{{\rm{O}}_{\rm{3}}}{\rm{ (}}l) {\rm{ }} + \frac{2}{3}{\rm{NO}}\left ( {{\rm{ g }}} \right) $ | (b) |
| $ {\Delta _{\rm{c}}}H_{\rm{m}}^\theta = {\Delta _{\rm{c}}}U_{\rm{m}}^\theta + \Delta {n_1}RT + \Delta {n_2}RT $ | (6) |
| $ \Delta n = \sum {n_i} ({\rm{ products}}, {\rm{g }}) {\rm{ }} - \sum {n_i} ({\rm{reactants}}, {\rm{g }}) $ | (7) |
where ∑ni is the total molar amount of gases in products or reactants.
The standard molar enthalpy of formation (
| $\begin{array}{l} {\Delta _{\rm{f}}}H_{\rm{m}}^\theta ({\rm{BNGM}}, {\rm{s}}) = {\rm{ }}3{\Delta _{\rm{f}}}H_{\rm{m}}^\theta {\rm{ (C}}{{\rm{O}}_{\rm{2}}}, {\rm{g}}) + \frac{{10}}{3}{\Delta _{\rm{f}}}H_{\rm{m}}^\theta ({{\rm{H}}_{\rm{2}}}{\rm{O}}, l) \\ + \frac{2}{3}{\Delta _{\rm{f}}}H_{\rm{m}}^\theta ({\rm{NO}}, {\rm{g}}) + {\rm{ }}\frac{4}{3}{\Delta _{\rm{f}}}H_{\rm{m}}^\theta ({\rm{HN}}{{\rm{O}}_{\rm{3}}}, l) \\ - {\Delta _{\rm{c}}}H_{\rm{m}}^\theta ({\rm{BNGM}}, {\rm{s}}) \end{array} $ | (8) |
The standard molar enthalpies of formation for CO2 (g), H2O (l), HNO3 (l) and NO (g) are as follows:
The standard molar enthalpy of combustion (
The test result indicates that impact sensitivity of BNGM is higher than 23.5 J. So, BNGM is relatively less sensitive. The sensitivity is close to that of parent material NG (> 30 J), but much lower than that of RDX (7.4 J) [29].
4 Conclusions(1) The thermal decomposition behavior of BNGM presents two exothermic decomposition processes. The self-accelerating decomposition temperature (TSADT) and critical temperature of thermal explosion (Tb) are 189.6 ℃ and 190.9 ℃, respectively.
(2) Specific heat capacity equation of BNGM is cp = 0.1763+3.2461×10-3T (285.0 K < T < 350.0 K), and the molar heat capacity is 251.9 J· mol-1 · K-1 at 298.15 K. Adiabatic time-to-explosion of BNGM is estimated to be about 280 s. The standard molar enthalpy of formation of BNGM is (-66.73±6.05) kJ·mol-1, and the impact sensitivity of BNGM is higher than 23.5 J. The thermal stability of BNGM is good.
| [1] |
Gao H X, Shreeve J M. Azole-based energetic salts[J]. Chemical Reviews, 2011, 111(11): 7377-7436. DOI:10.1021/cr200039c |
| [2] |
Ruan H W, Ling Y F, Wang G X, et al. Synthesis and characterization of 2 - nitro - 2 - azaadamantane - 4, 8 - diyl dinitrate[J]. Chinese Journal of Energetic Material (Hanneng Cailiao) , 2016, 24(6): 544-549. |
| [3] |
Li Y F, Wang M J, Xu K Z, et al. Thermal behaviors of 1-amino-2- nitroguanidine[J]. Chinese Journal of Energetic Material (Hanneng Cailiao) , 2016, 24(9): 848-852. |
| [4] |
Chavez D E, Parrish D A. Synthesis and characterization of 1- nitroguanyl-3-nitro-5-amino-1, 2, 4-triazole[J]. Propellants, Explosives, Pyrotechnics, 2012, 37(5): 536-539. DOI:10.1002/prep.v37.5 |
| [5] |
Shukla M K, Wang J, Seiter J. Understanding the fate of insen- sitive munitions compounds:computational study on adsorp- tion of nitroguanidine (NQ) and 1, 1-diamino-2, 2-dinitroeth- ylene (FOX-7) on pristine and Al-hydroxylated α-alumina sur- faces[J]. The Journal of Physical Chemistry C, 2017, 121(21): 11560-11567. DOI:10.1021/acs.jpcc.7b02938 |
| [6] |
Metelkina E L. 2 - Nitroguanidine derivatives:X. synthesis and nitration of 4-nitriminotetrahydro-1, 3, 5-oxadiazine and 2-ni- triminohexahydro-1, 3, 5 -triazine and their substituted deriva- tives[J]. Russian Journal of Organic Chemistry, 2007, 43(10): 1447-1450. |
| [7] |
Chavez D E, Hiskey M A, Gilardi R D. Novel high - nitrogen materials based on nitroguanyl - substituted tetrazines[J]. Organic Letters, 2004, 6(17): 2889-2991. DOI:10.1021/ol049076g |
| [8] |
Fu Q J, Zhu C H, Fu X Y. On the synthesis, structure and properties of spiro -nitramines and spiro -nitrosamines[J]. Acta Armamentarii, 1992, 1(1): 28-35. |
| [9] |
Zhang Q H, He C L, Yin P, et al. Insensitive nitrogen-rich ma- terials incorporating the nitroguanidyl functionality[J]. Chemistry-An Asian Journal, 2014, 9(1): 212-217. DOI:10.1002/asia.201301242 |
| [10] |
Li Y X, Wang J L, Wang Y H, et al. A novel synthetic method of 2-nitroimino-5-nitro-hexahydro-1, 3, 5-triazine (NNHT) [J]. Chinese Journal of Organic Chemistry, 2011, 31(2): 256-259. |
| [11] |
Jiao S X, Li T, Qi S Z. Synthesis of 2 - nitroiminimidazolidine[J]. Hebei Chemical Industry, 2002(6): 40. |
| [12] |
Kony M, Dagley I J. Synthesis of oetahydro-2, 5-bis (nitroimi- no) imidazo[4, 5 - d] imidazole[J]. Heterocycles, 1994, 38(3): 595-600. DOI:10.3987/COM-93-6597 |
| [13] |
Yu Y Z, Su Z, Duan B R, et al. Synthesis of polynitro compounds from nitroguanidine[J]. Propellants, Explosives, Pyrotechnics, 1989, 14(4): 150-152. DOI:10.1002/ (ISSN) 1521-4087 |
| [14] |
Li J K, Zhou C, Yang W, et al. Synthesis and characterization of 2, 4 -dinitrimino -1, 5 -dinitrohexahydro -1, 3, 5 -triazine[J]. Chinese Journal of Spectroscopy Laboratory, 2012, 29(4): 2540-2542. |
| [15] |
Zhou C, Wang B Z, Huo H, et al. A novel energetic material hydrazinium 3, 5-dinitroamino-1, 2, 4-triazole:synthesis and properties[J]. Chinese Journal of Energetic Materials (Hanneng Cailiao) , 2014, 22(4): 576-578. |
| [16] |
Li Y F, Zhai L J, Xu K Z, et al. Thermal behaviors of a novel nitrogen - rich energetic compound hydrazinium 3, 5 - dinitroamino-1, 2, 4-triazole[J]. Journal of Thermal Analysis Calo- rimetry, 2016, 126(3): 1-7. |
| [17] |
Kissinger H E. Reaction kinetics in differential thermal analysis[J]. Analytical Chemistry, 1957, 29(11): 1702-1706. DOI:10.1021/ac60131a045 |
| [18] |
Ozawa T. A method of analying thermogravimetric data[J]. Bulletin of the Chemical Society of Japan, 1965, 38(11): 1881-1886. DOI:10.1246/bcsj.38.1881 |
| [19] |
Hu R Z, Gao S L, Zhao F Q, et al. Thermal Analysis Kinetics[M]. 2th, Beijing: Science Press, 2008.
|
| [20] |
Zhang T L, Hu R Z, Xie Y, et al. The estimation of critical tem- peratures of thermal explosion for energetic materials using non - isothermal DSC[J]. Thermochimica Acta, 1994, 244(244): 171-176. |
| [21] |
Smith L C. An approximate solution of the adiabatic explosion problem[J]. Thermochimica Acta, 1975, 13(1): 1-6. |
| [22] |
Xu K Z, Song J R, Zhao F Q, et al. Thermal behavior, specific heat capacity and adiabatic time - to - explosion of G (FOX - 7) [J]. Journal of Hazardous Materials, 2008, 158(2-3): 333-339. DOI:10.1016/j.jhazmat.2008.01.077 |
| [23] |
Xu K Z, Song J R, Zhao F Q, et al. Non-isothermal decomposi- tion kinetics, specific heat capacity and adiabatic time-to-explosion of a novel high energy material:1-amino-1-methylami- no-2, 2-dinitroethylene (AMFOX-7) [J]. Journal of the Chinese Chemical Society, 2009, 56(3): 524-531. DOI:10.1002/jccs.v56.3 |
| [24] |
Ma H X, Yan B, Li Z N, et al. Preparation, non-isothermal decomposition kinetics, heat capacity and adiabatic time-to explosion of NTO · DNDZ[J]. Journal of Hazardous Materials, 2009, 169(1-3): 1068-1073. DOI:10.1016/j.jhazmat.2009.04.057 |
| [25] |
Xu K Z, Chen Y S, Wang M, et al. Synthesis and thermal be- havior of 4, 5-dihydroxyl-2- (dinitromethylene) -imidazolidine (DDNI) [J]. Journal of Thermal Analysis and Calorimetry, 2011, 105(1): 293-300. DOI:10.1007/s10973-010-1244-4 |
| [26] |
Vyzovkin S, Burnham A K, Criado J M, et al. ICTKA kinetics committee recommendations for performing kinetic computa- tions on thermal analysis data[J]. Thermochimica Acta, 2011, 520(1-2): 1-19. DOI:10.1016/j.tca.2011.03.034 |
| [27] |
Atkins P, Paula J D. Atkins' Physical Chemistry[M]. 7th, Beijing: High Education Press, 2006.
|
| [28] |
David R L. Handbook of chemistry and physics[M]. Boca Raton, FL: CRC Press, 2003.
|
| [29] |
Zhou T H, Li Y F, Xu K Z, et al. The new role of 1, 1-diamino- 2, 2 - dinitroethylene (FOX - 7):two unexpected reactions[J]. New Journal of Chemistry, 2017, 41(1): 168-176. DOI:10.1039/C6NJ03370A |

The thermal behavior, specific heat capacity, adiabatic time‐to‐explosion of bis (nitroguanidine) methane (BNGM) were studied by differential scanning calorimetry (DSC), micro‐DSC, thermogravimetry /differential thermogravimetry (TG/DTG) and its impact sensitivity was determined.




