2. Robust Munitions Center, CAEP, Mianyang 621999, China
2. 中国工程物理研究院安全弹药研发中心, 四川 绵阳 621999
By adding active metal powder, the power ability and explosion power of energetic materials can be improved quickly and effectively[1-2]. Al and B are used in propellants and explosives due to their high oxidation enthalpies, high combustion temperatures, and low molecular weight products.
Commonly, Al is widely recognized as one of the most ideal and suitable active metals with advantages of high oxidation heat, low price and wide source. Specially, the density and combustion heat of Al are 2.7 g · cm-3 and 31 kJ · g-1 in the view of thermodynamics respectively, while the density and combustion heat of B are 2.34 g · cm-3 and 59 kJ · g-1 re spectively[3]. Considering that the combustion heat of B is almost two times greater than that of Al, many scientists pay close attention to B. However, the melting point and boiling point of B are higher than that of Al, and the liquid B2O3 is formed in the early stage of combustion, the oxidation reaction of solid-liquid interfaces is obstructed, which result in that the ignition and combustion characteristics of B powder are poor, so that it is difficult to burn and release the high combustion heat completely. The ignition and combustion characteristics of the amorphous B powder were improved by using technologies of the oxidant AP coating B powder surface and the B-Al composite powder. Under the loading of detonation products of high-energy explosive, the higher combustion heat of the B-Al composite powder was released which have practical value.
B powder has been widely used in various kinds of propellants[4-8]. In recent years, there are some literatures published about metalized explosive containing B and Al powder. Underwater energy of DNTF based B-contained and Al-contained explosive was studied theoretically and experimentally, which indicated that the energy of B-contained explosive was better than that of Al-contained explosive[9-10]. Besides, better energy characteristics could be obtained if B and Al were used together. Pei et al.[11] studied the explosion performance of thermobaric fuel containing B, and the shock overpressure of the fuel containing B powder had no apparent advantage compared to the samples containing Al powder in the same measure point. While compared to the fuel containing Al powder, the fireball temperature of the fuel containing B sustained a long time. Recently, the thermal behaviors of various metallized RDX-based explosives with B powder were investigated by a thermogravimetric analyzer (TGA) and a high pressure differential scanning calorimeter(PDSC) technology, indicating that the RDX decomposition and its products could active B powder, and then made its oxidation or nitrogenation easy at high temperature[12]. Xu et al.[13-14] studied the combustion heat of the Al/B powder and its application in metallized explosives in underwater explosions. As the content of B powders was increased, the heat of combustion of the metal mixtures increased, and the combustion efficiency of B decreased.
As noted above, the addition of B and Al powders to explosives is a good way to enhance their blast effect, to improve the temperature of the explosion field and to prolong the duration of the higher temperature. However, the application of B in explosive is still at the exploratory stage at home and abroad. Thus, careful measurements are still needed to address specifically the mechanical sensitivity of metalized explosive containing B and Al powder to ensure the safety of preparation and characterization. In this work, a new HMX based metalized explosive containing B and Al powder, oxidizer AP, polyurethane binder was designed and prepared. The thermal properties, mechanical sensitivity, electrostatic spark sensitivity, cap initiation sensitivity, and ignition and propagation properties of the new metalized explosive were measured, which lay the foundation for the subsequent development of detonation reaction zone structure and power ability.
2 Experimental 2.1 Preparation 2.1.1 Raw MaterialsHMX:the content was 99.9%, and the particle size D50 are 6.7 μm, analytical reagent, and the particle sizes were Ф20-50 μm, Dalian potassium chlorate plant production. Al powder: the content was 99%, analytical reagent, and the particle sizes were Ф 1-5 μm, Liaoning Angang Industrial Fine Aluminum Powder Co. Ltd. production. B powder: the content was 99.9%, analytical reagent, and the particle sizes were Ф 1-5 μm, Hebei Baoding Pengda New Material Technology Co. Ltd. production. HTPB: molecular mass average was 2000, hydroxyl value was 0.76 mmol/g, Luoyang Liming Chemical Academy production.
2.1.2 Powder Mechanical Mixing MethodAccording to the designed formulation, nine kinds of pulverous metalized explosives listed in Table 1 and Fig. 1 consisted of HMX and AP or desensitized HMX and AP, metal powder were prepared. The photographs of three kinds of pulverous metalized explosives in Fig. 1 were taken with a Canon camera to observe the appearance of metalized explosives. Two kinds of B and Al compound powder used in this study were compound powder 1# and compound powder 2# that both had the mass radio of B and Al as 1:1. The difference was that the compound powder 1# was made by simple physical mixing method while the compound powder 2# was made by mechanical attrition method.

|
Fig.1 Photographs of three kinds of pulverous metalized explosives |
| Tab.1 Formulation of pulverous metalized explosive |
Through the processes of batching, kneading, vacuum casting and curing, four kinds of metalized explosives consisted of desensitized HMX or RDX, desensitized AP, B and Al compound powder, HTPB and TDI were prepared. The metalized explosive shown in Fig. 2 consisted of HMX and AP (60%), B powder (12%), Al powder (8%), and other additives including the HTPB binder (20%). The components of the three other metalized explosives were shown in Table 2.

|
Fig.2 Photograph of metalized explosive cylinder |
| Tab.2 Formulation of cast metalized explosive |
Scanning electron microscope(SEM) observations were carried out on Apollo300 SEM (Camscan Electron Optics, UK). When the voltage was 30 kV, the resolution was 1.2 nm. When the voltage was 1 kV, the resolution was 2.0 nm. The maximum magnification of the sample was 100 thousands. During the observation of appearance of micron metal powder samples, the magnifications were 0.5-20 thousands.
2.2.2 Thermal AnalysisThe DSC-TG experiments were performed by STA 449C DSC-TG and DSC 204 thermal analyzer (NETZSCH Co. German). The sample masses were (2 ± 0.50) mg, heating rate was 10 K · min-1, the experimental temperatures were 20-1000 ℃, the working atmosphere was nitrogen, the flow rate was 20 mL · min-1, and the pressure DSC was operated at 1 MPa.
2.2.3 Measurement of Sensitivity PropertiesThe mechanical sensitivity of metalized explosive was measured according to specifications of standardization methods GJB 772A-1997 601.1 (Impact sensitivity explosive probability method) and 602.1 (Friction sensitivity explosive probability method)[15]. The electrostatic spark sensitivity was determined by a model JGY-50 instrument. The sample masses were (20 ± 2) mg, the 50% firing voltage and 50% firing energy were calculated with the up-down method. In reference to the Chemical Physical Hazard Test Guide in the "test of cap initiation sensitivity 5(a)", the cap initiation sensitivity of sample was measured[16].
2.2.4 Plate Dent TestThe fiber tube of Ф 50 mm × 50 mm and 2 mm thick was filled with metalized explosive. In the plate dent test, the booster explosive was TNT cylinder of Ф 50 mm × 50 mm, the initiating system were PETN tablet and 8# industrial detonator, the identification plate was Q235 steel cylinder of Ф 100 mm × 40 mm.The detonation pressure was calculated through the dent depth of identification plate, and power ability of the metalized explosive were relatively evaluated[17-18].
3 Results and Discussions 3.1 Appearance of B and Al Compound PowderThe micro morphology of Al powder, B powder and two kinds of B and Al compound powders were observed by SEM. The SEM images at various scales are shown in Fig. 3.

|
Fig.3 SEM images of metal powder |
The Al powder is 1-5 μm spherical particles, and partial particles have agglomeration, as shown in Fig. 3. The structure of B powder is amorphous flake crystallite shape whose sizes are between 1-5 μm. For the B-Al compound powder, there are many little amorphous flake B powder on the surface of Al powder.
3.2 Thermal PropertiesThe TG-DSC curves at atmospheric pressure and DSC curves at atmospheric pressure or 1 MPa pressure of powdery AP, HMX, HMX+Al, and HMX+Al+B obtained at a heating rate of 10 K · min-1 are shown in Fig. 4.
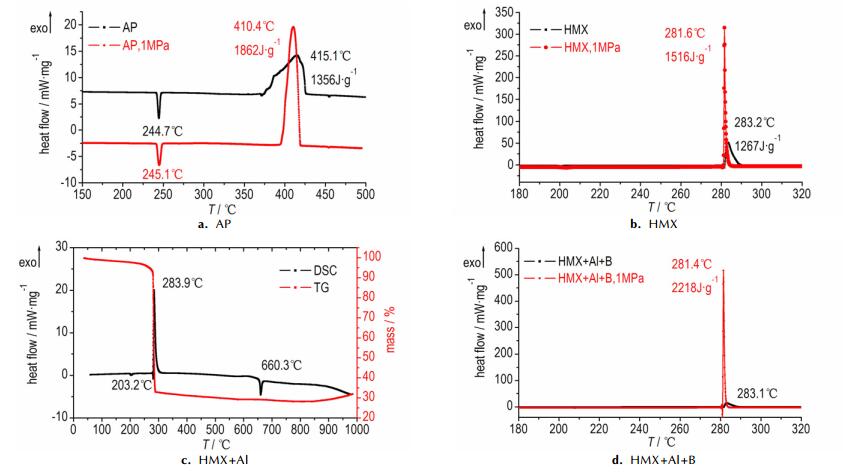
|
Fig.4 DSC and TG-DSC curves of AP, HMX, HMX+Al, and HMX+Al+B |
It can be seen from Fig. 4 that the endothermic peak of AP sample at about 245 ℃ is a crystalline transformation peak. The thermal decomposition of large AP particles (Φ > 200 μm) is different from that of small AP particles and there are two decomposition peaks for large AP particles[19]. In this paper, the AP sample is small particles (Φ=20-50 μm), so there is only one decomposition peak at temperature 410.4 ℃. The peak is wide and gentle at atmospheric pressure, while the exothermic peak is narrow and sharp and the released heat is also increased at 1 MPa pressure. The decomposition peak of HMX sample at 1 MPa pressure is slightly decreased and becomes very sharp. By adding Al powder in HMX, the crystalline transformation peak, the exothermic decomposition peak of HMX and the endothermic melting peak of Al powder are all slightly decreased. Under the conditions of room temperature to 1000 ℃ and N2 atmosphere, although the pressure have effects on thermal decomposition peak temperature of HMX and AP, Al powder and amorphous B powder occur to partial oxidation but no combustion.
3.3 SafetyThe safety of the metalized explosive containing B and Al powder was evaluated by sensitivity test, and the safety risk assessment for preparation and processing of kilogram grade sample was also evaluated. The mechanical sensitivity, electrostatic spark sensitivity and cap initiation sensitivity of six kinds of powdery metalize explosive are shown in Table 3.
| Tab.3 Sensitivities of pulverous metalized explosive |
The impact sensitivity, friction sensitivity and electrostatic spark sensitivity of the metalized explosives containing B and Al powder are 60%-80%, 100% and 3.83-6.40 kV, respectively, and the metalized explosive has cap initiation sensitivity, as shown in Table 3. The results show that the sensitivity of the metalized explosive containing B and Al powder is higher without binder, therefore the safety in the preparation and characterization test should be paid attention to. When the metalized explosive is impacted by drop hammer, it is often accompanied by a large noise and flash, due to the reaction energy release of burning metalized powder if explosion happens. Specially, for metalized explosive containing B and Al powder, the noise and flash are greater, the T10A steel sleeve is exploded into two parts, and the drop hammer is pushed up by the counterforce. The results indicate that the metalized explosive has strong after-effect power ability. When the metalized explosive is acted by friction hammer, it is often accompanied by a large noise and flash if explosion happens, which is only observed in the friction sensitivity test of CL-20 and BTF explosive[20-22].
Based on RDX or HMX, adding oxidizer AP, B-Al compound powder and binder HTPB, four kinds of metalized explosive containing B and Al powder were designed and prepared. Before curing, samples of 40 mg metalized explosive into the impact or friction devices, then pressed into tablet and put into the oven to curing. Afterwards, the mechanical sensitivity of metalized explosive was measured, the results are listed in Table 4.
| Tab.4 Mechanical sensitivities of cast metalized explosive |
It is shown that the impact sensitivity of the GR-1 formulation of RDX based explosive containing B powder is 88%, the friction sensitivity is 100%, as shown in Table 4, therefore the mechanical sensitivity of this metalized explosive is very high. In the case of other components unchanged, by replacing the B powder into B and Al compound powder, the impact sensitivity is decreased to 60%, and the safety requirements of preparation and processing of mixed explosive still can't been satisfied. Increasing the binder content of HTPB, the impact sensitivity of the GH-1 and GH-2 formulation of HMX based explosive containing B powder is less than 10%, the friction sensitivity is less than 30%, so that the safety requirements for the preparation and processing technology of mixed explosive are satisfied.
3.4 Ignition and propagation propertiesThe plate dent test of nine kinds of metalized explosives, powdery HMX and AP, TNT and cast metalized explosive cylinder of Ф 50 mm were done. Device of plate dent test are shown in Fig. 5, photographs of some identification plate are shown in Fig. 6, and results of plate dent test for metalized explosive are shown in Table 5.

|
Fig.5 Device of plate dent test |

|
Fig.6 Photographs of some identification plate |
| Tab.5 Results of plate dent test for metalized explosive |
It is shown that the identification plate has an obvious dent, which indicates that the design and preparation of metalized explosives have better ignition and propagation properties, as shown in Fig. 6 and Table 5. By companion, it is found that the greater the density of explosive, the deeper of dent is. When the quality of metal powder Al:B is 1:1, the dent is not the deepest. The detonation pressure of TNT, HMX and AP at different densities is calculated by using VLW code, and the linear relationship between the dent depth and the detonation pressure is shown in Fig. 7. The linear equation is
| $p = 10.0714 + 1.2591h$ | (1) |
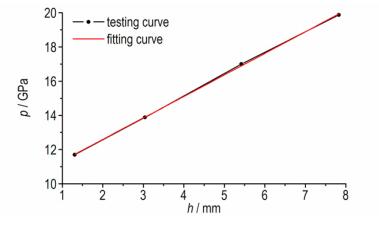
|
Fig.7 Curves of relationship between dent depth and detonation pressure |
where, h is the dent depth of identification plate, mm; p is the detonation pressure, GPa.
In addition, the cap initiation sensitivity and detonation ability test of metalized explosive were carried out. About 700 g JS-2-1 metalized explosive was put into the fiber tube of 80 mm diameter. The charge density is 0.9415 g · cm-3. The identification plate above steel rim is Q235 steel plate of 1 mm thick while the identification plate below steel rim is Q235 steel plate of 8mm thick, as shown in Fig. 8a. The test results show that metalized explosive has cap initiation sensitivity, and the Q235 steel plate of 1 mm thick is exploded into pieces, and the Q235 steel plate of 8 mm thick is exploded with a large hole of more than 80 mm diameter, as shown in Fig. 8b, indicating that the metalized explosive has great after-effect power ability.
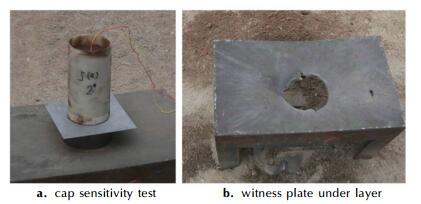
|
Fig.8 Cap sensitivity test of metalized explosive |
(1) For investigation of the thermal properties, mechanical sensitivity, electrostatic spark sensitivity, cap initiation sensitivity, ignition and propagation properties of metalized explosives, the HMX based metalized explosives containing B and Al are designed and prepared by powder mechanical mixing method and cast PBX's method.
(2) From room temperature (25 ℃) to 1000 ℃ and under N2 atmosphere, pressures have effect on thermal decomposition peak temperature of HMX and AP, while Al powder and amorphous B powder occur to partial oxidation but no combustion.
(3) The mechanical sensitivities of the powdery metalized explosives are so high that the safety requirements for preparation and processing technology of mixing explosive cannot be satisfied although it can be obviously lowered by adding desensitized HMX and AP. While for the cast metalized explosives including mass compositions 35% of metal powder, 20% of AP, 60% of HMX and AP, 20% of HTPB and other additives satisfy the safety requirements.
(4) The nine kinds of metalized explosives of Ф 50 mm containing B and Al powder prepared all have cap initiation sensitivity and can propagate stable detonation, showing strong after-effect power ability. According to the experimental results, the detonation parameters and after-effect ability of metalized explosive can be improved by increasing the casting density.
| [1] | Krier H, Burton R, Pitman S, et al. Shock initiation of crystalline boron in oxygen and fluorine compounds[J]. Journal of Propulsion and Power, 1996, 12(4): 672-679. DOI:10.2514/3.24088 |
| [2] | Balas W, Nicolich S, Capellos C, et al. New aluminized explosives for high energy, high blast warhead applications[C]//Proceedings 2006 Insensitive Munitions & Energetic Materials Technology Symposium, Bristol, UK, 2006. |
| [3] | LI Shu-fen. Boron-based solid fuels[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 1995, 3(2): 1-8. |
| [4] | Mohan G, Williams F A. Note on laser-ignited boron(Boron ignition and combustion mechanisms based on high speed photographs of laser ignited boron particles)[C]//American Institute of Aeronautics and Astronautics, Aerospace Sciences Meeting, 10th, USA, 1972. |
| [5] | Dreizin E L, Keil D G, Felder W. Phase changes in boron ignition and combustion[J]. Combustion and Flame, 1999, 119(3): 272-290. DOI:10.1016/S0010-2180(99)00066-8 |
| [6] | Flower P Q, Steward P A. Improving the efficiency of metallized explosives[C]//Symposium on Energetic Materials and Insensitive Munitions, USA, 2006. |
| [7] | Luman J R, Wehrman B, Kuo K K, et al. Development and characterization of high performance solid propellants containing nano-sized energetic ingredients[J]. Proceedings of the Combustion Institute, 2007, 31(2): 2089-2096. DOI:10.1016/j.proci.2006.07.024 |
| [8] | Macek A, Semple J M. Combution of boron particles at atmospheric pressure[J]. Combustion Science and Technology, 1969, 1(3): 181-191. DOI:10.1080/00102206908952199 |
| [9] | WANG Hao, WANG Qin-hui, JIN Da-yong, et al. Underwater energy of DNTF based boron-contained and aluminum-contained explosive[J]. Chinese Journal of Explosive and Propellants, 2007, 30(6): 38-40. |
| [10] | FENG Xue-song, ZHAO Sheng-xiang, DIAO Xiao-qiang, et al. Experimental research of underwater energy of explosives containing boron/metal[J]. Chinese Journal of Explosive and Propellants, 2009, 32(5): 21-24. |
| [11] | PEI Ming-jing, MAO Gen-wang, ZHENG Kai-wei, et al. Explosion performance of thermobaric fuel containing boron[J]. Chinese Journal of Explosive and Propellants, 2006, 29(4): 1-4. |
| [12] | REN Xiao-ning, SHAO Ying-hui, LIU Zi-ru, et al. Thermal behavior of boron metalized RDX-based explosives[J]. Chinese Journal of Explosive and Propellants, 2012, 35(3): 47-51. |
| [13] | S Xu, Yu Chen, X Chen, et al. Combustion heat of the Al/B powder and its application in metallized explosives in underwater explosions[J]. Combustion, Explosion, and Shock Waves, 2016, 52(3): 342-349. DOI:10.1134/S001050821603014X |
| [14] | CHEN Yan, XU Sen, WU De-jun, et al. Experimental study of the explosion of aluminized explosives in air[J]. Central European Journal of Energetic Materials, 2016, 13(1): 117-134. DOI:10.22211/cejem/64967 |
| [15] | GJB772A-1997, Explosive test methods[S]. Beijing: Press of national defense industry, 1997. |
| [16] | Recommendations on the transport of dangerous goods, Manual of tests and criteria[M]. United Nations New York and Geneva, 2009. |
| [17] | ZENG Gui-yu, HUANG Hui, GAO Da-yuan, et al. Effects of additives on detonator initiation sensitivity and work capacity of ANFO[J]. Explosion and Shock Waves, 2008, 28(5): 467-470. |
| [18] | DONG Hai-shan, ZHOU Fen-fen. Performance of high explosives and correlates[M]. Beijing: Science Press, 1989. |
| [19] | LIU Zi-ru, YIN Cui-mei, KONG Yang-hui, et al. The thermal decomposition of ammonium perchlorate[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2000, 8(2): 75-79. |
| [20] | WANG Cai-ling, ZHAO Sheng-xiang. Mechanical sensitivity of AP with different particle size[J]. Chinese Journal of Explosive and Propellants, 2006, 29(6): 27-29. |
| [21] | Simpson R L, Urtiew P A, Ornellas D L, et al. CL-20 performance exceeds that of HMX and its sensitivity is moderate[J]. Propellants, Explosives, Pyrotechnics, 1997, 22(5): 249-255. DOI:10.1002/(ISSN)1521-4087 |
| [22] | McGuire R R. Properties of benzotrifuroxan[R]. California University, Livermore (USA). Lawrence Livermore Laboratory, 1978. |




