2. School of Chemical Engineeing, Jiangsu University, Zhenjiang 212013, China;
3. Sichuan Petroleum Perforating Materials Co.LTO, Neijiang 642177, China;
4. Xi′an Modern Chemistry Research Institute, Xi′an 710065, China;
5. School of Mechanical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
2. 江苏大学化学化工学院, 江苏 镇江 212013;
3. 四川石油射孔器材有限责任公司, 四川 内江 642177;
4. 西安近代化学研究所, 陕西 西安 710065;
5. 南京理工大学机械学院, 江苏 南京 210094
In recent decades, extensive attention has been paid on high quality energetic material(EM) with high energy, high density, high heat resistance, and low sensitivity. The high quality EM was extensively used in the area of advanced conventional weapons, rocket propellants and explosives. There is tremendous interest in developing efficient methods to synthesize energetic complexes in recent years. Energetic complexes refer to series of complexes with high explosive performances, and have attracted considerable interest especially in primary explosives and energetic catalysts in pyrotechnic and propellant mixtures due to their potential properties, such as high energy, good fluidity, low sensitivities and good catalytic performance on the thermal decomposition of ammonium perchlorate (AP) and 1, 3, 5-trinitrohexahydro-1, 3, 5-triazine (RDX)[1-9].
2, 6-Diamino-3, 5-dinitropyridine-1 -oxide (ANPyO) and 2, 4, 6-triamino-3, 5-dinitropyridine-1-oxide (TANPyO)[10] are realistic, high-performance energetic materials that are thermally stable and insensitive to shock, spark and friction[11], with similar performance, stability and sensitivity to that of 2, 4, 6-triamino-1, 3, 5-trinitrobenzene (TATB). They belong to a multi-amino, multi-nitro-heterocyclic N-oxide with structure units —N+—O- and —NH2. It may form stable complexes with a large number of metal ions similar to quinoxaline N1, N4-dioxide[12-13]. In accordance with previous studies on metal complexes of quinoxaline N1, N4-dioxide derivatives, we selected metals including Cu(Ⅱ)[14], Co(Ⅲ)[15], Fe(Ⅲ)[15], Pb(Ⅱ)[16] to construct novel ANPyO-based coordination compounds with similar structures to metal complexes of quinoxaline N1, N4-dioxide derivatives. The asymmetric unit of these metal complexes comprises one central metal canon and two or three deprotonated ANPyO anions. Each central metal canon has a distorted octahedron, coordinated by nitrogen and oxygen from deprotonated ANPyO. This unique coordination mode that ligands direct coordinate with metal ions without additional anions or cations can stabilize entire molecular complexes. Therefore, metal complexes of ANPyO exhibit good thermal stability, high density and low sensitivity, with some properties similar to ANPyO. Moreover, TG-DTG and DSC results show that these complexes have significant catalytic effects on the thermal decomposition of ammonium perchlorate(AP)[14-16]. A lot of applications have been proposed for metal complexes of ANPyO, such as insensitive explosive, propellant and energetic catalyst.
Basedon our previous studies on metal complexes of ANPyO, we speculate that TANPyO can form energetic complexes with a large number of transition metal ions due to its similar structure and properties to ANPyO. Compared with transition metal ions i.e Cu(Ⅱ), Co(Ⅲ), Fe(Ⅲ), Ni(Ⅱ), the Pb(Ⅱ) ion [16] has large ion radius, variable coordination numbers, and diverse coordination geometries. The Pb(Ⅱ) ion also has a tendency to form stable framework structure with a large number of ligands. Furthermore, it is discovered that the lead salts of energetic compounds possess better catalytic effect and ability to reduce pressure exponent for the propellant.
This study develops a new strategy for the synthesis and characterization of Pb(Ⅱ) complex of TANPyO (Pb(TANPyO)) and report its structure, sensitivity performances, thermal decomposition behavior and catalytic properties on the thermal decomposition of AP.
2 Experimental 2.1 Materials and InstrumentsAll chemicals used were analytical grade, and purchased from commercial sources without further purification. IR spectra were recorded on a Nicolet-10 infrared spectrophotometer (Nicolet Company, USA) over the frequency range 4000-500 cm-1 using the KBr pellet technique. Elemental analyses were performed with an Elementar vario EL Ⅲ microanalyzer (Elmentar Analysen Systeme GmbH, Germany).
DSC studies were performed on a DSC823eMETTLER TOLEDO with heating rates of 2.5, 5, 10, 20 K·min-1. TG-DTG analysis was conducted on TGA/SDTA851eMETTLER TOLEDO under a nitrogen atmosphere at a heating rate of 10 K·min-1, at a flow rate of 30 mL·min-1.
The friction sensitivity was measured by applying a Julius Peter apparatus following the BAM method[17]. Impact sensitivity was determined with the Bruceton method on a standard fall hammer apparatus, and the compacted sample was hit with a 2.5 kg drop hammer on the apparatus[18]. Shock sensitivity was determined on a designed shock sensitivity apparatus[19].
2.2 Synthesis 2.2.1 Synthesis of TANPyOTANPyO was prepared according to literature [10]. Anal. Calcd.(%): C 26.09, H 2.61, N 36.52. Found: C 26.12, H 2.65, N 36.48.
2.2.2 Synthesis of Pb(C5H4N6O5)Pb(CH3COO)2·3H2O(0.380 g, 1.0 mmol) was added to a solution of TANPyO (0.230 g, 1.0 mmol) in ethanol (20 mL) at 80 ℃ for 2 h, then cooled to room temperature and filtered, washed with ethanol and dried in air. An orange-yellow solid powder (0.381 g) formed with yield of 87.50%(based on TANPyO). m.p. 311-312 ℃. Calcd: Pb 47.61, C 13.79, H 0.92, N 19.31. Found: Pb 47.68, C 13.81, H 0.89, N 19.33. The molecular formula of Pb(TANPyO) is Pb(C5H4N6O5).
3 Results and Discussion 3.1 FTIR SpectraThe FTIR spectra of TANPyO and Pb(TANPyO) are illustrated in Fig. 1. The main vibration bands related with coordination are shown in Table 1.

|
Fig.1 FTIR spectra of TANPyO and Pb(TANPyO) |
| Tab.1 Main IR bands of TANPyO and Pb(TANPyO) |
The FTIR spectrum of Pb(TANPyO) in Fig. 1 shows a similar pattern with those previously reported for metal complexes in the family of quinoxaline N1, N4-dioxide [12-13]. Two strong bands corresponding to νas(NH2) and νs(NH2) of the amino group of 2-position and 6-position in the 3431 and 3362 cm-1 region for the TANPyO, disappear after coordination. Only one band (ν(NH)) with medium intensity at 3304 cm-1 is observed, it is in agreement with the presence of a secondary amine. The νas(NH2) and νs(NH2) of the amino group of 4-position in the 3314 and 3253 cm-1 region, for the TANPyO shift to medium after coordination, without a significant displacement. The strong ν (N—O) at 1284 cm-1 for the TANPyO, shifts to medium after coordination, without a significant displacement too. As previously reported, this behavior supports the coordination of the TANPyO to Pb(Ⅱ) through the N—O group and the deprotonated amino group.
3.2 Sensitivity TestsTo understand the stability and hazardous property of Pb(TANPyO), their sensitivity properties are measured, and compared with the sensitivity ones of TATB.The results are listed in Table 2.
| Tab.2 Sensitivity test results for TANPyO and Pb(TANPyO) |
TANPyO belongs to a multi-amino, multi-nitro-heterocyclic compound with symmetry structure. The intramolecular and intermolecular hydrogen bonds are formed by the amino and nitro groups [10]. The molecular structure of TANPyO is planar, which is similar to that of TATB. The extensive intramolecular and intermolecular hydrogen bonds can result in high crystal density, thermal stability and insensitive to impact and friction. As shown in Table 2, the impact sensitivity, friction sensitivity and shock wave sensitivity of TANPyO and TATB are 300 cm, 36 kg, 5.2 mm and 320 cm, 36 kg, 4.5 mm, respectively, showing that TANPyO and TATB are insensitive.
It is believed that theintramolecular and intermolecular hydrogen bonds, the π-electron conjugated effect and the amino donor effect are responsible for the low sensitivity of TANPyO. When TANPyO forms the energetic complex with Pb(Ⅱ), the crystal structure of the energetic complex relative to TANPyO experiences two alterations. On one hand, when the crystal structure of the energetic complex changes from the TANPyO plane layered structure to the three-dimensional network structure, the intramolecular and intermolecular hydrogen bonds become weak. This is not conducive to reducing sensitivity of the Pb(Ⅱ) complex. On the other hand, TANPyO coordinates with Pb(Ⅱ) directly, which is unique, can stabilize the entire molecular complex and reduce the sensitivity of the Pb(Ⅱ) complex. The change in sensitivity of the Pb(Ⅱ) complex relative to TANPyO is the combined results of above-mentioned two aspects.
As shown in Table 2, the impact sensitivity, friction sensitivity and shock wave sensitivity of Pb(TANPyO) is 305 cm, 36 kg and 4.9 mm, respectively, showing that in comparison with TANPyO, the sensitivities of Pb(TANPyO) are decreased. From above analysis, we consider that the form of coordinate bonds and chelation are the main reasons of lower sensitivity for the Pb(Ⅱ) complex.
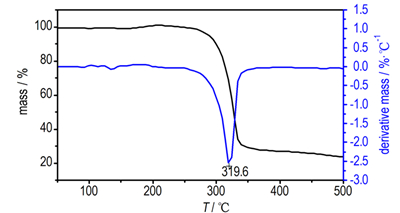
3.3 Thermal DecompositionDSC and TG-DTG determinationsare conducted to identify the thermal behavior of Pb(TANPyO). TG-DTG and DSC curves for Pb(TANPyO) at a heating rate of 10 K·min-1 are shown in Fig. 2 and Fig. 3, respectively.
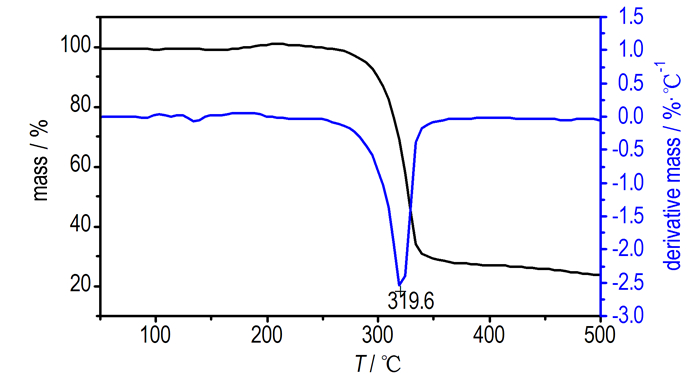
|
Fig.2 TG-DTG curves of Pb(TANPyO) |
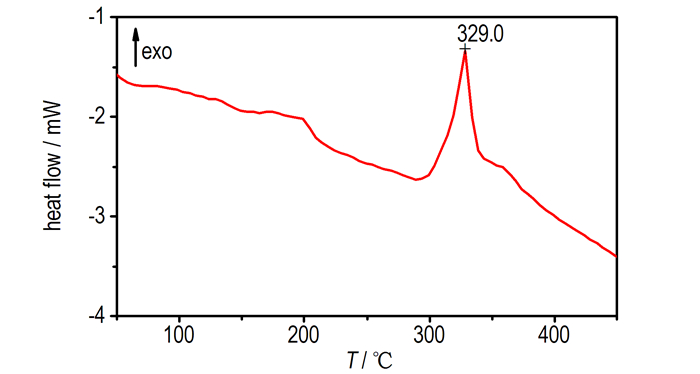
|
Fig.3 DSC curve of Pb(TANPyO) |
TG-DTG curves of Pb(TANPyO) is divided into two stages. The first stage is a fast mass-loss process, with 72.1% mass loss from the initial mass in the temperature range of 229.2-361.4 ℃, which reaches the largest rate at 319.6 ℃, The DSC curve of Pb(Ⅱ) complex shows that there is an exothermic process in the first stage, in the range of 291.6-355.9 ℃. A sharp exothermic peak is shown in the DSC curve with a peak temperature of 329.0 ℃. The first stage is the Pb-O, Pb-N bonds breaking and the ring breaking of TANPyO in the temperature range of 210.5-361.4 ℃. The second stage is a slow mass-loss process, with 4.1% mass loss from the initial mass in the temperature range of 361.4-500.0 ℃. The DSC curve of Pb(TANPyO) shows that there is no obvious change in the second stage. The mass fraction of the final residue is 23.8%.
3.4 Non-isothermal Kinetics AnalysisWe studied the kinetic parameters of the first exothermic process of the complex by using Kissinger′s [20] and Ozawa-Doyle′s[21-22] methods. The Kissinger equation and Ozawa-Doyle equations are as follows:
| $ \begin{array}{l} \ln \frac{\beta }{{{T_{\rm{p}}}^2}}=\ln \left( {\frac{{RA}}{E}} \right)-\frac{E}{{R{T_{\rm{p}}}}}\\ \lg \beta + \frac{{0.4567E}}{{R{T_{\rm{p}}}}} = C \end{array} $ |
WhereTp is the peak temperature, ℃; R is the gas constant, 8.314 J·mol-1·K-1; β is the linear heating rate, K·min-1; C is a constant. Based on the multiple non-isothermal DSC curves obtained at four different heating rates of 2.5, 5, 10, 20 K·min-1, the values of the apparent activation energy (EK and EO) (where subscript K: Kissinger′s method: subscript O: Ozawa-Doyle′s method), the pre-exponential factor (AK) and linear correlation coefficient (rK and rO) of the two intense exothermic decomposition processes were determined by Kissinger′s and Ozawa-Doyle′s methods. The detailed data and the calculated kinetic parameters are listed in Table 3.
| Tab.3 Non-isothermal reaction kinetic parameters for Pb(TANPyO) |
The calculated results using both methods are within the normal range of the kinetic parameters of such thermal decomposition reaction, and correspond well with each other. Therefore, the Arrhenius equation of the exothermic decomposition process can be expressed as lnk=66.25-331.9x103/(RT).
3.5 Effects on the Thermal Decomposition of APAP is the common oxidizer in composite solid propellants, and the thermal decomposition characteristics of AP greatly influenceon the combustion behavior of solid propellants [23-24]. In order to provide theoretical support to further performance study as combustion catalysts, the Pb(TANPyO) is explored as a promoter to the thermal decomposition of AP. The catalytic effect of Pb(TANPyO) on the thermal decomposition of AP (Pb(TANPyO) and AP are mixed in a mass ratio of 1:4) is investigated by TG-DTG and DSC measurements at a heating rate of 10 K·min-1 under N2 atmosphere in the range of 50-500 ℃. The results obtained are shown in Fig. 4, Fig. 5 and Fig. 6.
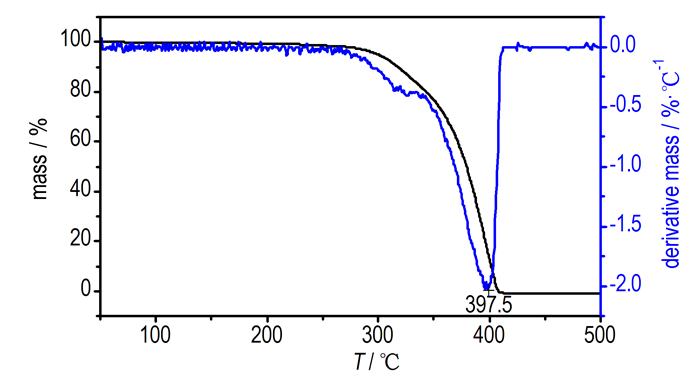
|
Fig.4 TG-DTG curves of pure AP |
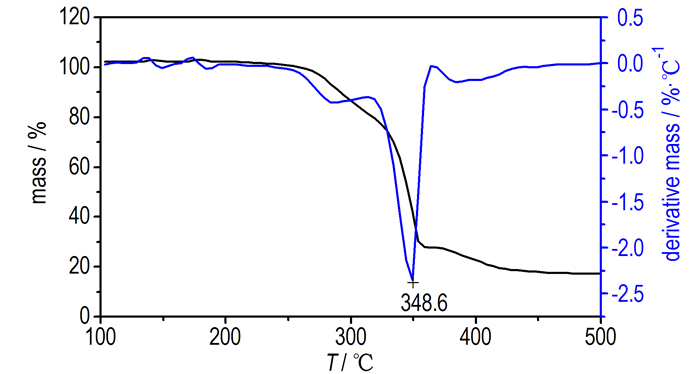
|
Fig.5 TG-DTG curves of AP with 20% Pb(TANPyO) |
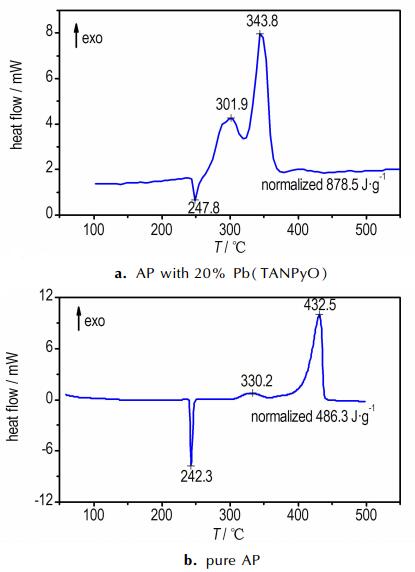
|
Fig.6 DSC curves of pure AP and AP with 20% Pb(TANPyO) |
TG-DTG curves of pure AP and AP with 20% Pb(TANPyO) are shown in Fig. 4 and Fig. 5, respectively. As shown in Fig. 4, the thermal decomposition of pure AP occurs in two mass-loss steps. The 21% mass-loss at low temperature of from 264.3 ℃ to 345.1 ℃ is attributed to the partial decomposition of AP. The 79% mass-loss at high temperature of 345.1℃ to 409.7 ℃ is caused by the complete decomposition of the intermediate to volatile products. The TG and DTG curves for the thermal decomposition of AP in the presence of Pb(TANPyO) are shown in Fig. 5. The thermal decomposition of AP catalyzed by Pb(TANPyO) shows that there are no noticeable changes in the decomposition pattern. AP is completely decomposed at the lower temperature of 348.6 ℃ in a shorter time.
The DSC curves for pure AP and AP in the presence of Pb(TANPyO) are also shown in Fig. 6. The endothermic peak at 242.3 ℃ is due to a crystallographic transition. The exothermic peak at 330.2 ℃ and 432.5 ℃ in Fig. 6 is attributed to the low-temperature decomposition (LTD) process and high-temperature decomposition (HTD) process of AP, corresponding to the two mass loss steps. The DSC curve of AP in the presence of Pb(TANPyO) shows that Pb(TANPyO) additive has no effects on the crystallographic transition temperature, but significant changes in the decomposition pattern.
The exothermic band of the mixed system of Pb(TANPyO) with AP has two broad peak, revealing a complicated mechanism of decomposition. In comparison with pure AP, the high-temperature decomposition peak of the mixed system is shifted 88.7 ℃ downwards, the low-temperature decomposition peak of the mixed systems is shifted 28.3 ℃ downwards and the decomposition heat of the mixed system is increased by 392.2 J·g-1, indicating that AP decomposition is accelerated in the presence of Pb(TANPyO).
4 Conclusions(1) The energetic Pb(TANPyO) is synthesized and characterized, its molecular formula is determined as Pb(C5H4N6O5).
(2) Impact sensitivity, friction sensitivity and shock wave sensitivity of the complex is 305 cm, 36 kg and 4.9 mm, respectively.
(3) The peak temperature of the complex decomposition reaction is 329.0 ℃, having a better heat-resistance ability.
(4) The complex makes the low temperature exothermic decomposition peak and high temperature exothermic decomposition peak of AP decrease by 28.3 ℃ and 88.7 ℃, respectively, and the heat of decomposition of AP increase by 392.2 J·g-1, showing that the complex has significant catalytic effects on the thermal decomposition of AP.
Acknowledgement: We gratefully acknowledge the financial support from Nanjing University of Science and Technology and Xi′an Modern Chemistry Research Institute.| [1] |
Jones D E, Armstrong K, Parekunnel T, et al. The thermal behaviour of BTAW, a high nitrogen fuel[J]. Journal of Thermal Analysis and Calorimetry, 2006, 86(3): 641-649. DOI:10.1007/s10973-006-7719-7 |
| [2] |
Friedrich M, Galvez-Ruiz J C, Klapotke T M, et al. BTA copper complexes[J]. Inorganic Chemistry, 2005, 44(22): 8044-8052. DOI:10.1021/ic050657r |
| [3] |
Singh R P, Verma R D, Meshri D T, et al. Energetic nitrogen rich salts and ionic liquids[J]. Angewandte Chemie International Edition, 2006, 45(22): 3584-3601. DOI:10.1002/(ISSN)1521-3773 |
| [4] |
ZOU M, JIANG X, LU L, et al. Nano or micro? A mechanism on thermal decomposition of ammonium perchlorate catalyzed by cobalt oxalate[J]. Journal of Hazardous Materials, 2012, 225: 124-130. |
| [5] |
Shvedenkov Y, Bushuev M, Romanenko G, et al. Synthesis and characterization of an energetic compound Cu(Mtta)2(NO3)2 and effect on thermal decomposition of ammonium perchlorate[J]. Journal of Hazardous Materials, 2011, 197: 199-203. DOI:10.1016/j.jhazmat.2011.09.074 |
| [6] |
Karaghiosoff K, Klapotke T M, Mayer P, et al. Salts of methylated 5-aminotetrazoles with energetic anions[J]. Inorganic Chemistry, 2008, 47: 1007-1019. DOI:10.1021/ic701832z |
| [7] |
Liu H B, Jiao Q Z, Zhao Y, et al. Mixed oxides derived from Cu—Co layered double hydroxide nanorods: Preparation, characterization and their catalytic activities[J]. Journal of Alloys and Compounds, 2010, 496(1-2): 317-323. DOI:10.1016/j.jallcom.2010.02.004 |
| [8] |
Kaiser M, Ticmanis U. [N2H5]2+[N4C—N=N—C—N4]2-: A new high-nitrogen high-energetic materia[J]. Inorganic Chemistry, 2001, 40: 3570-3575. DOI:10.1021/ic010063y |
| [9] |
Shvedenkov Y, Bushuev M, Romanenko G, et al. Magnetic anisotropy of new layered copper(Ⅱ) bromide complexes of 1-substituted tetrazoles[J]. European Journal of Inorganic Chemistry, 2005, 167: 1678-1682. |
| [10] |
Hollins R A, Merwin L H, Nissan R A, et al. Aminonitropyridines and their N-Oxides[J]. Journal of Heterocyclic Chemistry, 1996, 33: 895-904. DOI:10.1002/jhet.v33:3 |
| [11] |
CHENG J. Synthesis, characterize and properties of insensitive explosive about pyridine derivatives[D]. Nanjing; Nanjing University of Science and Technology, 2012.
|
| [12] |
Carolina U, Marisol V, Maria H T. Cytotoxic palladium complexes of bioreductive quinoxaline N1, N4-dioxideprodrugs[J]. Bioorganic & Medicinal Chemistry, 2009, 17: 1623-1629. |
| [13] |
Belen T M, Carolina U, Antonio M. Design against of novel iron tuberculosis compounds as potential therapeutic agents[J]. Journal of Inorganic Biochemistry, 2010, 104: 1164-1170. DOI:10.1016/j.jinorgbio.2010.07.005 |
| [14] |
Liu J J, Liu Z L, Cheng J. Synthesis, crystal structure and properties of a novel tetra-nuclear Cu complex of ANPyO[J]. Journal of Solid State Chemistry, 2013, 197: 198-203. DOI:10.1016/j.jssc.2012.08.007 |
| [15] |
Liu J J, Liu Z L, Cheng J, et al. Synthesis, crystal structure and properties of energetic complexes constructed from transition metal cations (Fe and Co) and ANPyO[J]. RSC Advances, 2013, 3(9): 2917-2923. DOI:10.1039/c2ra22839d |
| [16] |
Liu J J, Liu Z L, Cheng J. Synthesis, crystal structure and catalytic effect on thermal decomposition of RDX and AP: An energetic coordination polymer[Pb2(C5H3N5O5)2(NMP)·NMP]n[J]. Journal of Solid State Chemistry, 2013, 200: 43-48. DOI:10.1016/j.jssc.2013.01.014 |
| [17] |
Meyer R, Kohler J. Explosives[M]. 4th revised and extended. New York: VCH publishers, 1993: 197.
|
| [18] |
Dixon W J, Mood A M. A method for obtaining and analyzing sensitivity Data[J]. Journal of the American Statistical Association, 1948, 43: 109-126. DOI:10.1080/01621459.1948.10483254 |
| [19] |
Liu Z T, Lao Y L. Initiating explosive experimental[M]. Beijing: Beijing institute of technology, 1995, 138.
|
| [20] |
Kissinger H E. Reaction kinetics in differential thermal analysis[J]. Analytical Chemistry, 1957, 29(11): 1702-1706. DOI:10.1021/ac60131a045 |
| [21] |
Ozawa T. Chem. A new method of analyzing thermogravimetric data[J]. Bulletin of the Chemical Society of Japan, 1965, 38: 1881-1886. DOI:10.1246/bcsj.38.1881 |
| [22] |
Doyle C D. Kinetic analysis of thermogravimetric data[J]. Journal of Applied Polymer Science, 1961, 5(15): 285-290. DOI:10.1002/app.1961.070051506 |
| [23] |
Liu H B, Jiao Q Z, Zhao Y, et al. Mixed oxides derived from Cu-Co layered double hydroxide nanorods: Preparation, characterization and their catalytic activities[J]. Journal of Alloys and Compounds, 2010, 496(1-2): 317-323. DOI:10.1016/j.jallcom.2010.02.004 |
| [24] |
Xia Z Q, Chen S P, Wei Q, et al. Syntheses and characterization of energetic compounds constructed from alkaline earth metal cations (Sr and Ba) and 1, 2-bis(tetrazol-5-yl)ethane[J]. Journal of Solid State Chemistry, 2011, 184(7): 1777-1783. DOI:10.1016/j.jssc.2011.05.017 |
An energetic Pb(Ⅱ) complex of TANPyO was synthesized, and its structure and properties were characterized with FT-IR spectroscopy, elemental analysis, sensitivity tests, TG-DTG and DSC.




