Furazanyl ether compounds have been becoming an important research direction in recent years as high energetic explosives and plasticizers due to their good performances [1-4]. FOF-13 is a new furazanyl ether energetic material firstly reported by Sheremetev[5] from 3,3′-dicyanodifurazanyl ether, and it exhibits a melting point of 48 ℃, a high density of 1.97 g·cm-3, a decomposition temperature above 270 ℃ and a standard enthalpy of formation (ΔHf) -146.3 kJ·mol-1[1-3]. However, the synthesis conditions and other properties are few reported.
In order to investigate FOF-13 thoroughly, a novel five-step synthetic route was designed(Scheme 1) in the first time, and FOF-13 and its intermediates were characterized by IR, 13C NMR, 19F NMR and elemental analysis. At the same time, the main energetic properties of FOF-13 were also studied.
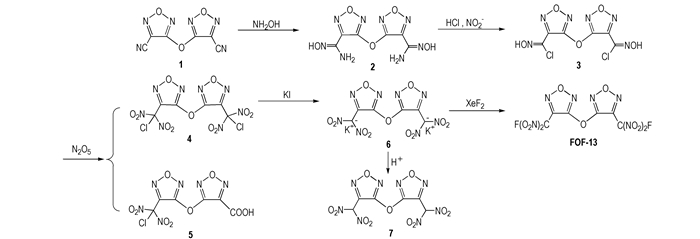
|
Scheme1 A novel synthetic route of FOF-13 |
1H NMR, 13C NMR and 19F NMR were obtained on a Bruker AV500 NMR spectrometer. Elemental analyses (C, H and N) were performed on a VARI-El-3 elementary analysis instrument. Infrared spectra were obtained from KBr pellets on a Nicolet NEXUS870 Infrared spectrometer in the range of 4000~400 cm-1. Differential scanning calorimetry (DSC) studies were carried out on a Q200 apparatus (TA, USA) with heating rates of 10 K·min-1, using dry oxygen-free nitrogen as atmosphere with a flowing rate of 50 mL·min-1. The TG-DTG experiment was performed with a SDT-Q600 apparatus (TA, USA) operating at a heating rate of 10 K·min-1 in a flow of dry oxygen-free nitrogen at 100 mL·min-1.
The impact and friction sensitivities of FOF-13 were determined with a ZBL-B impact sensitivity instrument and a MGY-2 friction sensitivity instrument (Nachen, China), respectively. The mass of fall hammer is 2 kg. The swing angle and gauge pressure is 60°. The sample used for each test is about 20 mg. The detonation velocity (Dexp) of FOF-13 was investigated with the GJB772A-1997 702.1. FOF-13 (18 g) was pressed into a cylinder (Φ=20 mm), and the loading density (ρ) was 1.69 g·cm-3.
3,3′-Dicyanodifurazanyl ether (FOF-2) was prepared according to the published procedures [6]. Other chemicals were obtained from commercial sources and used without further purification.
2.3 Synthesis 2.3.1 Synthesis of 3,3′-bis(amidoximino)difurazanyl ether (2)To a mixture of water (50 mL), isopropanol (25 mL), 3,3′-dicyanodifurazanyl ether (1) (4.16 g, 20.0 mmol) and hydroxylamine hydrochloride (2.85 g, 41 mmol), sodium carbonate anhydrous was added in batches, and then the reaction mixture was stirred at room temperature for 1 h. The precipitate was filtered, washed with ice water, and dried in vacuo to obtain a white solid 5.02 g with a yield of 91.2%. m.p. 203~204 ℃. 1H NMR (DMSO-d6, 500 MHz): δ=10.67 (s, 2H, OH), 6.28 (s, 4H, NH2). 13C NMR (DMSO-d6, 125 MHz): δ=160.31 (C—O), 142.23 (C—C=N), 141.33 (C—NH2). IR (KBr, ν/ cm-1): 3495, 3454, 3349, 3172, 2919, 1680, 1656, 1525, 1101, 1021, 969. Calc. for C6H6N8O5: C 26.67, N 41.18, H 2.24; Found: C 26.62, N 41.07, H 2.18.
2.3.2 Synthesis of 3,3′-bis(chloroximido)difurazanyl ether (3)Appropriate above compound 2 (5.40 g, 20.0 mmol) was dissolved in 55 mL of concentrated hydrochloric acid and 30 mL of water at room temperature. Saturated sodium nitrite (2.38 g, 41.0 mmol) in water was added dropwise to a stirred solution of amide oxime. After stirring for 2 h at 273 K, the reaction mixture was heated to 293 K for 1.5 h until N2 evolution stopped. The resulting white precipitate was filtered, washed with water, recrystallized from MeOH/H2O (1:1) and dried in vacuo to yield white solid 5.40 g with a yield of 87.5%. m.p. 60~61 ℃. 1H NMR (DMSO-d6, 500 MHz): δ=13.71 (s, 2H, OH). 13C NMR (DMSO-d6, 125 MHz): δ=158.99 (C—O), 143.03 (C—C=N), 123.40 (C—Cl). IR (KBr, ν/cm-1): 3533, 3167, 3020, 1570, 1518, 1126, 1024, 942, 657. Calc. for C6H2N6O5Cl2: C 23.32, N 27.20, H 0.65; Found: C 23.30, N 27.15, H 0.68.
2.3.3 Synthesis of 3,3′-bis(chlorodinitromethyl)difurazanyl ether (4) and 3-chlorodinitro-methyl-3′-carboxyl difurazanyl ether (5)To a suspension of the above compound 3 (0.55 g, 1.8 mmol)in 50 mL of CHCl3 at 293 K was added N2O5 (2.2 g, 20 mmol). The mixture was heated to 318 K and kept at this temperature for 40 min. The solvent was evaporated and the residue was subjected to column chromatography on silica gel to isolate colorless crystals 4 (0.23 g, 30.0 %) and 5 (0.25 g, 41.6 %). Compound 4 : m.p. 68-69 ℃. 13C NMR (DMSO-d6, 125 MHz): δ=157.67 (C—O), 140.37 (C—C=N), 112.75 (CCl(NO2)2). IR (KBr, υ/ cm-1): 1613, 1582, 1515, 1291, 1049, 971. Calc. for C6N8O11Cl2: C 16.72, N 26.00%; Found: C 16.82, N 25.94%. Compound 5 : m.p. 127-128 ℃. 1H NMR (DMSO-d6, 500 MHz): δ=13.84 (s, 1H, OH). 13C NMR (DMSO-d6, 125 MHz): δ=160.74, 139.82, 133.01, 124.66, 113.58, 106.14. IR (KBr, ν/cm-1): 3140, 2916, 2675, 1752, 1620, 1605, 1580, 1510, 1269, 1131, 1030, 982. Calc. for C6HN6O9Cl: C 21.41, H 0.30, N 24.97; Found: C 21.38, H 0.41, N 24.86.
2.3.4 Synthesis of potassium salt of 3,3′-bis(dinitromethyl)difurazanyl ether (6)Compound 4 (1.0 g, 2.3 mmol) was dissolved in MeOH (8 mL) and treated with solution of KI (1.5 g, 9.0 mmol) in MeOH (15 mL) at room temperature. The resulting mixture was stirred at room temperature for 1 h and triturated with Et2O (20 mL). Precipitate was collected, washed with ice-cold water, MeOH, and Et2O to furnish a yellow solid (0.81 g, 85.7%). m.p. 98 ℃ (loss of H2O), 245 ℃ (dec., DSC measurement, 10 K·min-1). 13C NMR (DMSO-d6, 125 MHz): δ=160.77 (C—O), 142.31 (C—C=N), 118.67 (C—(NO2)2). IR (KBr, ν/cm-1): 1589, 1526, 1479, 1239, 1070, 997. Calc. for C6N8O11K2: C 16.44, N 25.57; Found: C 16.15, N 25.36.
2.3.5 Synthesis of 3,3′-bis(dinitromethyl)difurazanyl ether (7)Compound 6 (0.65 g, 1.5 mmol) was suspended in water (3 mL), and then acidified with 50% sulfuric acid (1 mL) at room temperature. The mixture was extracted with diethyl ether (3×10 mL), dried over MgSO4, and the solvent was evaporated to obtain compound 7 (0.44 g, 81.5 %). m.p. 68-69 ℃. 1H NMR (DMSO-d6, 500 MHz): δ=10.49 (s, 2H, CH). 13C NMR (DMSO-d6, 125 MHz): δ=161.22 (C—O), 142.77 (C—C=N), 119.10 (CH(NO2)2). IR (KBr, ν/cm-1): 3004, 2983, 1623, 1585, 1524, 1481, 1366, 1322, 1239, 1148, 1037, 999. MS (ESI) m/z: 360.98 [M-H]-.Calc. for C6H2N8O11: C 19.90, H 0.56, N 30.94; Found: C 19.74, H 0.73, N 30.78.
2.3.6 Synthesis of 3, 3'-bis(fluorodinitromethyl)difurazanyl ether (FOF-13)To a suspension of compound 6 (0.6 g, 1.37 mmol) in anhydrous acetonitrile at 20 ℃, XeF2 (0.92 g, 5.50 mmol) was added. After the mixture was stirred for 48 h at 20 ℃, the acetonitrile was evaporated, and the residue was treated with some water to afford many colorless crystals (0.23 g, 42.6%). m.p. 43.5 ℃ (DSC measurement, 10 K·min-1); 13C NMR (DMSO-d6, 125 MHz): δ=158.41 (s, C—O), 137.00 (d, J13C-19F=25, C—C=N), 113.88 (d, J13C-F19=294.0 Hz, CF(NO2)2). 19F NMR (DMSO-d6, 470.5 MHz): δ=-106.29. IR (KBr, ν/cm-1): 1616, 1576, 1515, 1310, 1196, 1138, 1048, 981. Calc. for C6N8O11F2: C 18.10, N 28.15%; Found: C 18.26, N 27.92%.
3 Physical chemistry properties and detonation performances for FOF-13Some main energetic properties of FOF-13 were determined or calculated, and listed in Table 1. The results show that the mean detonation velocity is 8497 m·s-1 with the standard deviation of 160. Moreover, we also speculated the detonation velocity (Dcalc=9300 m·s-1) based on the maximum density obtained from the crystal structure.
| Tab.1 Physical chemistry and detonation properties of FOF-13 |
An excellent energetic plasticizer FOF-13 was synthesized via a novel five-step reaction process, and its structure was fully characterized. FOF-13 exhibits low melting point (43.5 ℃). Its impact sensitivity (15 J) and friction sensitivity (64%) are superior to RDX. The detonation velocity for FOF-13 (Dexp=8497 m·s-1, ρ=1.69 g·cm-3) were measured to show a high energy, making it suitable as a promising high energy plasticizer for solid propellant.
| [1] |
Sheremetev A B, Kulagina V O, Aleksandrova N S, et al. Dinitro trifurazans with oxy, azo and azoxy bridges[J]. Propellants Explosives Pyrotechnics, 1998, 23(3): 142-149. DOI:10.1002/(ISSN)1521-4087 |
| [2] |
Sheremetev A B, Kharitonova O V, Mantseva E V, et al. Nuclephilic displacement in furazan series Ⅲ: Reactions with O-Nucleophiles[J]. zh, Org Khim, 1999, 35: 1525-1537. |
| [3] |
Sheremetev A B, Kharitonova O V, Mel'nikova TM, et al. Synthesis of symmetrical difurazanyl ethers[J]. Mendeleev Commun, 1996, 4(6): 141-143. |
| [4] |
Sheremetv A B, Mantseva E V. Hydroxyfurazans: outlook to using[C]//32th International Annual Conference of ICT, Karlsruhe, Germany, 2001, 103: 1-5.
|
| [5] |
Sheremetev A B. 3, 3-Bis(1-fluoro-1, 1-dinitromethyl)difurazanyl ether[C]//29th International Annual Conference of ICT, Karlsruhe, Germany, 1998, 58: 1-6.
|
| [6] |
FAN Yan-jie, B. Z. WANG Bo-zhou, LAI Wei-peng, et al. Synthesis, characterization and quantum chemistry study on 3, 3'-dicyanodifurazanyl ether (FOF-2)[J]. Chinese Journal Organic Chemistry, 2009, 29(4): 614-620. |
| [7] |
Gao H, Joo Y-H, Parrish D A, et al. 1-Amino-1-hydrazino-2, 2-dinitroethene and corresponding salts: synthesis, characterization and thermolysis studies[J]. Chem Eur J, 2011, 17(16): 4613-4618. DOI:10.1002/chem.201002858 |
| [8] |
Eaton P E, Gilardi R L, Zhang M-X. Polynitrocubanes: advanced high-density, high-energy materials[J]. Advance Materials, 2000, 12(15): 1143. DOI:10.1002/(ISSN)1521-4095 |
| [9] |
Karl K, Renato R. Development of an efficient to manufacture bis (2-fluoro-2, 2-diniroethyl) formal (FEFO)[C]//18th International Annual Conference of ICT, Karlsruhe, Germany, 1987, 28: 1-5.
|
| [10] |
Rothstein L R. Predicting high explosive detonation velocities from their composition and structure (Ⅱ)[J]. Propellants Explosives Pyrotechnics, 1981, 6(4): 91-93. DOI:10.1002/(ISSN)1521-4087 |
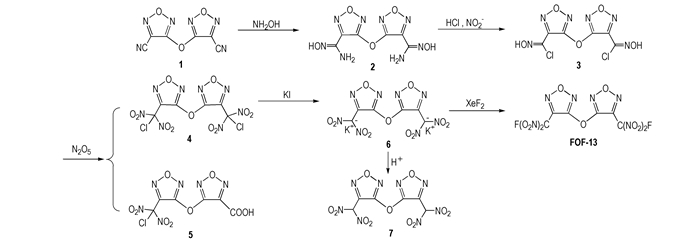
An excellent energetic plasticizer FOF-13 was synthesized via a novel five-step reaction process, and its main energetic properties were determined.




