A Novel Synthesis Route of [1, 2, 5]Oxadiazolo [3, 4-e] [1, 2, 3, 4]tetrazine-4, 6-Di-N-oxide
1 Introduction
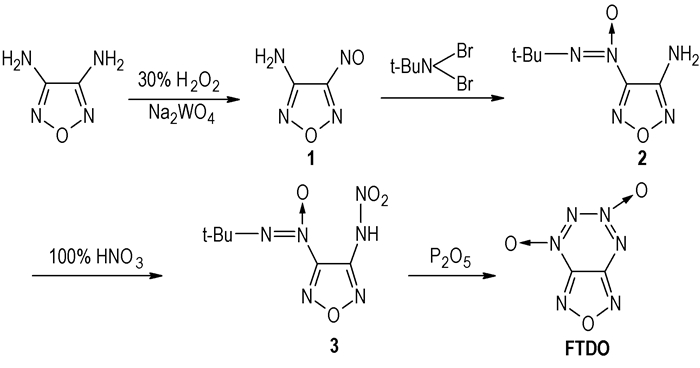
An energetic material, [1, 2, 5] oxadiazolo [3, 4-e] [1, 2, 3, 4] tetrazine-4, 6-di-$N$-oxide (FTDO), whose density (tested) was 1.85 g·$\text{cm}^{-3}$[2], enthalpy of formation was 1010 kcal·$\text{kg}^{-1}$[3], calculated detonation velocity pressure was 9802 m·$\text{s}^{-1}$, and detonation pressure was 44.78 GPa, was firstly synthesized and reported by Russian scientists in 1995[1]. The five-step synthetic route of FTDO, which was synthesized from 3, 4-diaminofurazan (DAF) via the reactions of oxidation, amino protection, condensation, hydrolysis and cyclization with a total yield of 14.63%, had been reported [1]. However, there were many shortcomings in that synthetic route, including reaction conditions rigorous, difficult separation and purification of FTDO and expensive $\text{NO}_{2}\text{BF}_{4}$ reagent.
A novel synthetic route of [1, 2, 5] oxadiazolo[3, 4-e][1, 2, 3, 4] tetrazine 4, 6-di-$N$-oxide(FTDO) was designed and FTDO was synthesized from 3, 4-diaminofurazan(DAF) via the reactions of oxidation, condensation, nitrification and cyclization with a total yield of 30.89%(Scheme 1). FTDO and its intermediates were characterized by $^{13}\text{C}$ NMR, $^{14}\text{N}$ NMR, $^{15}\text{N}$ NMR, $^{17}\text{O}$ NMR, IR, MS and EA etc. Compared with the five-step route in reference, this new route simplifies the synthetic route with milder reaction conditions, more convenient separation and purification of FTDO and lower-cost reagents, which offers potential for application in industry.
2 Synthesis of 3-amino-4-nitrosofurazan (1)
A mixture of benzene(200 mL), 30% hydrogen peroxide (145 mL, 1.29 mol) and sodium tungstate dihydrate (16.5 g, 0.05 mol), concentrated sulfuric acid (10 mL, 180 mmol) being added dropwise at 5~10 ℃, and then DAF(1), was stirred at 15 ℃ for 1.5 h. The organic layer was separated, washed with water and dried by anhydrous magnesium sulfate. After the drying agent being filtered, the solvent was removed to obtain 5.19g yellow solid, yield 91.05%. m.p. 76~78 ℃. $^{1}\text{H}$ NMR (DMSO, 500 MHz)$δ$: 6.86(s, 2H); IR(KBr, $\text{cm}^{-1}$)ν: 3421, 3318, 1628, 1531, 1482, 1409, 1284, 1014; Anal. Calcd for $\text{C}_{2}\text{H}_{2}\text{N}_{4}\text{O}_{2}$: C 21.06, H 1.77, N 49.12; Found: C 20.82, H 1.77, N 48.95.
3 Synthesis of 3-amino-4-(tert-butyl-NNO-azoxy) furazan (2)
A suspension solution of $N$, $N$-dibromo-tert-butylamine(11.5 g, 50 mmol), CuCl(10 g, 100 mmol), compound $\boldsymbol{(1)}$(0.57 g, 50 mmol) in methylene chloride (500 mL) was stirred at 15~25 ℃ for 15 h. The reaction mixture was poured into ice-water (500 mL), and then sodium thiosulfate was added. The organic layer was separated, washed with water and dried by anhydrous magnesium sulfate, filtered and the solvent was removed to obtain 6.8 g yellow solid, yield 73.50%. m.p. 156~158 ℃. $^{1}\text{H}$ NMR (DMSO, 500 MHz)$δ$: 1.43(s, 9H), 6.59(s, 2H); $^{13}\text{C}$ NMR (DMSO, 500 MHz)$δ$: 25.06, 59.99, 150.77, 151.55; IR(KBr, $\text{cm}^{-1}$)ν: 3472, 3356, 2980, 1624, 1566, 1453, 1367, 1183; MS(EI) $m$/$z$(%): 185($\text{M}^{+}$, 5), 112(20), 102 (20), 57(100), 41(35), 29(28); Anal. Calcd for $\text{C}_{6}\text{H}_{11}\text{N}_{5}\text{O}_{2}$: C 38.92, H 5.99, N 37.82; Found: C 39.36, H 5.96, N 35.60.
4 Synthesis of 3-nitramino-4-(tert-butyl-NNO-azoxy) furazan (3)
Pure nitric acid (2.72 g, 21.6 mmol) was added to a stirred and cooled (0 ℃) solution of carbon tetrachloride (2 g, 10.8 mmol). After being warmed to room temperature gradually, the mixture was stirred for 2 h and the solvent was removed by vacuum. Methylene chloride(100 mL) was added, the solution was washed with ice-water (20 mL) and aqueous layer was extracted with methylene chloride(2×50 mL).The combined organic layer was dried by anhydrous magnesium sulfate, filtered and the solvent was removed to obtain 2.47 g yellow solid, yield 99.54%. m.p. 92~94 ℃; $^{1}\text{H}$ NMR ($\text{CDCl}_{3}$, 500 MHz)$δ$:1.52($t$, $J$=64 Hz, 9H), $δ$:11.34(s, 1H); $^{13}\text{C}$ NMR ($\text{CDCl}_{3}$, 500 MHz)$δ$: 25.34, $δ$ 61.90, $δ$:142.91, 150.35; IR(KBr, $\text{cm}^{-1}$)ν:3291, 2983, 1621, 1510, 1488, 1315, 1164;MS (EI) $m$/$z$(%) EI : 230 ($\text{M}^{+}$, 40), 212(97), 172(35), 154(28), 119(18), 98(20); Anal. Calcd for $\text{C}_{6}\text{H}_{1}\text{N}_{6}\text{O}_{3}$: C 31.30, H 4.35, N 36.52; Found: C 31.73, H 4.41, N 36.12.
5 Synthesis of [1, 2, 5] oxadiazolo [3, 4-e] [1, 2, 3, 4] tetrazine-4, 6-di-$N$-oxide (FTDO)
At the temperature of 0 ℃, the solution of 3-nitramino-4-(tert-butyl-NNO-azoxy) furazan (1 g, 4.35 mmol) in acetonitrile(160 mL) was added to phosphoric anhydride(20 g), and then keep this temperature for 0.5 h. After being warmed to 55 ℃, the mixture was stirred for 5 h and the solvent was poured into ice-water(200 mL).The product was extracted with methylene chloride(10×20 mL) and dried by anhydrous magnesium sulfate, filtered and the solvent was removed to obtain 0.70 g yellow oil, the yellow oil was decolorized with active floridin and obtain 0.315 g bright yellow crystal, yield 46.37%. m.p. 109~111 ℃; 13C NMR (acetone-$d_{6}$, 500 MHz)$δ$: 156.81, 144.71; $^{14}\text{N}$ NMR (acetone-$d_{6}$, $\text{MeNO}_{2}$ as the standard, 500 MHz)37.6(ν$_{1/2}$=519 Hz)(N-1, 3), 10.0(ν$_{1/2}$=458 Hz)(N-5), -42.2 (ν$_{1/2}$=9 Hz) (N-6), -50.7(ν$_{1/2}$=9 Hz)(N-4), -104.7(ν$_{1/2}$=279 Hz)(N-7); $^{15}\text{N}$ NMR(acetone-$d_{6}$, $\text{MeNO}_{2}$ as the standard, 500 MHz)35.6, 38.8(N-1, 3), -42.3(N-6), -50.9(N-4), -104.7(N-7); $^{17}\text{O}$ NMR (acetone-$d_{6}$, $\text{MeNO}_{2}$ as the standard, 500 MHz) 532.3(ν$_{1/2}$=251 Hz), 504.2(ν$_{1/2}$=186 Hz), 441.7(ν$_{1/2}$=441 Hz); IR(KBr, $\text{cm}^{-1}$)ν:1589, 1543, 1514, 1460, 1418, 1317, 1148, 1018, 939; MS (EI) $m$/$z$(%): 156($\text{M}^{+}$, 25), 84 (4), 68 (45), 52 (13), 30 (100); Anal. Calcd for $\text{C}_{2}\text{N}_{6}\text{O}_{3}$:C 15.38, N 53.47; Found: C 15.38, N 53.85.
6 Performances of physical and detonation for FTDO
The performances of physical, such as density, melting point and dissolubility, were obtained by test, detonation velocity and detonation pressure were calculated by VLW method[4]. The datas were shown in the Table 1.
表 1(Tab. 1)

Tab. 1 The performances of FTDO
| properties |
results |
test condition |
| appearance |
yellow crystal |
eyeballing |
| density/g·$\text{cm}^{-3}$ |
1.85 |
experimental method |
| melting point/℃ |
110~112 |
melting point apparatus |
| nitrogen content/% |
52.47 |
elemental analysis |
| dissolubility |
soluble in $\text{CH}_{2}\text{Cl}_{2}$
and $\text{CH}_{3}\text{COCH}_{3}$ |
experimental method |
| detonation velocity/m·$\text{s}^{-1}$ |
9802
($d$=1.85 g·$\text{cm}^{-3}$) |
VLW method |
| detonation pressure/GPa |
44.78 |
VLW method |
enthalpy of formation
/kcal·$\text{kg}^{-1}$ |
1010 |
reference[3] |
|
Tab.1 The performances of FTDO |
A new type of energetic material, [1, 2, 5]oxadiazolo[3, 4-e][1, 2, 3, 4]tetrazine-4, 6-di-$N$-oxide(FTDO), was synthesized by a novel route, and its performances of physical and detonation were tested or calculated by VLW method. The results showed that FTDO was a new type of energetic material with a novel structure, and would have the potential for application in industry.
Using 3,4-diaminofurazan as a primary material,a new energetic material [1,2,5]oxadiazolo[3,4-e][1,2,3,4]tetrazine-4,6-di-N-oxide(FTDO) was synthesized via the reaction of oxidation,condensation,nitrification and cyclization with a total yield of 30.89%,FTDO and its intermediates were characterized by NMR,IR,MS and elemental analysis.