2. Shandong Machinery Group Co., LTD., Zibo 255201, China
2. 山东机器集团有限公司,山东 淄博 255201
2, 4, 6-trinitro-1, 3, 5-trihydroxybenzene (trinitrophloroglucinol, TNPG) belongs to the polynitro hydroxybenzenes. It has three nitrogroups and three phenolic hydroxyl groups conjugated with the ring[1-7]. It is an important explosive. It has been used in the chemical industry as an ingredient for making dyes and in explosive industry as an ingredient for primer compositions, percussion caps, and detonator for mulations[8]. TNPG is a strong acidic organic compound so it can form a large number of salts with metals which are efficient primary explosives and used in important applications in the military and commercial industry. Alkalina and alkali-earth metal salts of TNPG as well as Zn, Cd, Pb, Mn, Ag and ammonium salts have been synthesized and extensively studied[9-11]. Till now, the enthalpy of dissolution of TNPG dissolved in N, N-dimethylformamide (DMF) and alcohol (EtOH) are studied in our research group[12], but enthalpy of solution of TNPG dissolved in deionized water and the thermodynamic properties of the solutions have rarely been reported.
In this paper, the process of dissolution of TNPG dissolved H2O and thermochemistry and thermodynamic properties are studied at different concentrations by a SETARAMC 80 calorimeter at 298.15 K. The empirical formulas of enthalpy of dissolution, the values of standard molar enthalpy of dissolution, relative apparent molar enthalpy of dissolution and relative partial molar dissolution enthalpy are all determined. The kinetic formula was calculated. From the formulas we can deduce the rate constant and order of the reaction.
2 Experiment 2.1 Preparation of reagents and compoundsThe compound TNPG is synthesized according to the reported paper[5]. The molecular structure and the thermal decomposition mechanism of TNPG have been studied by using differential scanning calorimetry(DSC), thermogravimetry-derivative thermogravimetry(TG-DTG), and FTIR technologies. It was purified and dried. Before the experiment it was sifted through a 200-mesh sieve, and then kept in a dryer for 24 h. The solvent, H2O used in the experiment was deionized with an electrical conductivity of 6.25×10-8 S·cm-1.
2.2 Equipment and conditionsA microcalorimetry, SETARAM C80 calorimeter (Setaram Instrumentation, Caluire, France) was used in the experiment at (298.15±0.01) K. It has two cells, one for reaction cell, the other for reference cell to cancel out the effect of residual deviation. Both the cells are made of stainless steel. The temperature is measured by 100 pairs of 100 Ω thermocouples located around the two vessels and it is held constant to within 0.01 K during each measurement. The microcalorimeter was calibrated by Joule effect and the sensitivity was obtained to be 30.501 μV·mW-1. The solution and solvent which are separated with a membrane made by polytetrafluoroethylene (PTEF, 0.05 mm thick) are put into the reaction cell.
The enthalpy of dissolution of KCl (special purity) in deionized water was tested for checking the accuracy of the calorimeter. The experimental result of the enthalpy of dissolution for KCl is 17.2225 kJ·mol-1 while the reported result is (17.241±0.018) kJ·mol-1[13]. The data from the calorimeter is reliable.
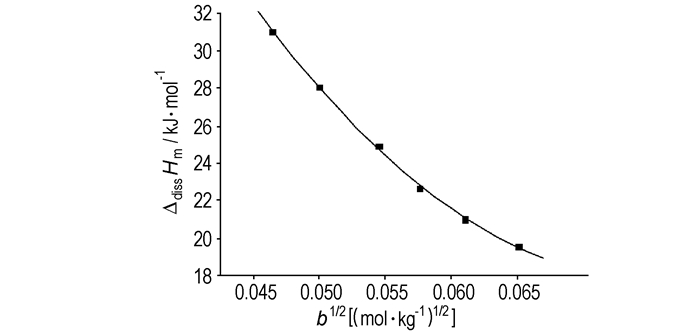
3 Results and discussions 3.1 Thermochemistry of TNPG dissolved in H2OSix times measurements with different concentrations were picked up in the experiment. The quantity of the solvent H2O was definite to be 3.0 mL while the quantity of TNPG was varied. The molalities and their molar dissolution enthalpy (ΔdissHm) are shown in Table 1. The relationship between the concentration and the ΔdissHm are shown in Fig. 1.
| Tab.1 Enthalpy of dissolution of TNPG in H2O at 298.15 K |
|
Fig.1 The relationship between the concentration and the ΔdissHm of TNPG in H2O at 298.15 K |
Based on the reported empirical formula Eq. (1)[14].
| $ {{\Delta }_{\rm{diss}}}H=A+Bb+C{{b}^{1/2}} $ | (1) |
Where, b is the molality of solution, mol·kg-1; A, B and C are the regression coefficients.
The empirical formula of TNPG dissolved in H2O are obtained by fitted the experimental data in Fig. 1.
| $ {{\Delta }_{\rm{diss}}}{{H}_{\rm{m}}}=106.79926+15538.58581\ b{-}2352.19194\ {{b}^{1/2}} $ | (2) |
The standard enthalpy of dissolution ΔdissHθm(b=0)are calculated:
| $ {{\Delta }_{\rm{diss}}}H_{\rm{m}}^{\theta }=106.799\ \rm{kJ}\cdot \rm{mo}{{\rm{l}}^{\rm{-1}}} $ | (3) |
The formulas of the relative apparent molar enthalpy of dissolution (ФLi) and the relative partial molar enthalpy (Li) were also identified according to the reported empirical formulas Eq. (4) and Eq. (5)[14].
| $ \mathit{\Phi }{{\mathit{L}}_{\mathit{i}}}={{\Delta }_{\rm{diss}}}H\left( b=b \right)-{{\Delta }_{\rm{diss}}}H\left( b=0 \right) $ | (4) |
| $ {{L}_{i}}=b\left[\frac{\partial {{\Delta }_{\rm{diss}}}H}{\partial b} \right]+\mathit{\Phi }{{L}_{i}} $ | (5) |
The formulas of ФLi and Li are as follows:
| $ \mathit{\Phi }{{L}_{i}}=15538.5858\ b-2352.19194\ {{b}^{1/2}} $ | (6) |
| $ {{L}_{i}}=62154.34324\ b-2328.28791\ {{b}^{1/2}} $ | (7) |
It can be deduced that the process of TNPG dissolving in H2O is endothermal. It is because that the interaction among the molecules of solute is stronger than the interaction between the molecules of solute and solvent. And as the concentration increases, the quantity of heat reduces in the process. The maximum is in the infinite dilution, which is known as the standard enthalpy of dissolution, the value ΔdissHθm=106.799 kJ·mol-1.
3.2 Thermodynamics of TNPG dissolved in H2OThe heat flow curves with different concentrations recorded by using a microcalorimeter at 298.15 K are similar in tendency of TNPG dissolved in H2O. The reactions are endothermal. The rate constant (k) and the reaction order (n) are irrelevant with concentrations. The original data from the curve were shown in Table 2.
| Tab.2 Thermokinetic data of TNPG dissolved in H2O at 298.15 K |
Based on Equation (8) derived from the literature[15], the experimental data are fitted by a linear least squares method.
| $ \ln \left[\frac{1}{{{H}_{\infty }}}\frac{{\rm d}{{H}_{i}}}{{\rm d}t} \right]=\ln k+n\ln \left[1-\frac{{{H}_{i}}}{{{H}_{\infty }}} \right] $ | (8) |
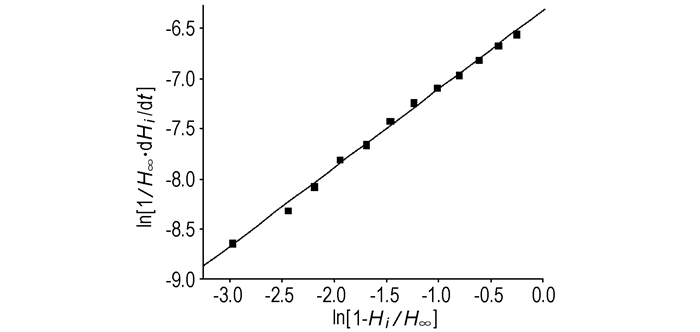
Where, H∞ is the total reaction enthalpy, kJ·mol-1; Hi is the reaction heat in a certain time, kJ·mol-1; k is the rate constant of reaction; n is the reaction order. The fitting results are shown in Fig. 2.
|
Fig.2 The kinetic curve of TNPG dissolved in H2O |
The curve shown in Fig. 2 can be received. From the data of Table 2 the kinetic formula of TNPG dissolved in H2O is obtained:
| $ \ln \left[\frac{1}{{{H}_{\infty }}}\frac{{\rm d}{{H}_{i}}}{{\rm d}t} \right]=-6.32176+0.78377\ln \left[1-\frac{{{H}_{i}}}{{{H}_{\infty }}} \right], \ \ \ \ {{R}^{*}}\rm{=}0.9967 $ | (9) |
Where, R* is correlation coefficient.
From the parameters of the kinetic formula the rate constant of reaction (k) and the reaction order (n) can be calculated: n=0.78377, k=1.80×10-3 s-1.
4 Conclusions(1) The empirical formula for the dissolution enthalpy of TNPG dissolved in H2O at 298.15 K is determined, as well as the standard molar enthalpy of dissolution (ΔdissHθm), the relative apparent molar enthalpy of dissolution (ФLi) and the relative partialmolar enthalpy (Li) are calculated respectively. The value ΔdissHθm=106.799 kJ·mol-1.
(2) The process of TNPG dissolving in H2O is endothermal. It is because that the the interaction among the molecules of solute is stronger than interaction between the molecules of solute and solvent. And as the concentration increases, the quantity of heat reduces in the processes. The maximum is in the infinite dilution, which is known as the standard enthalpy of dissolution ΔdissHθm(b= 0).
(3) On the basis of the experimental data and calculated results, the rate constant of reaction (k) and the reaction order(n) are also determined: n=0.78377, k=1.80×10-3 s-1.
| [1] |
WANG Li-qiong, CHEN Hong-yan, ZHANG Tong-lai, et al. Synthesis, characterization, thermal and explosive properties of potassium salts of trinitrophloroglucinol[J]. Journal of Hazardous Materials, 2007, 147(1-2): 576. DOI:10.1016/j.jhazmat.2007.01.043 |
| [2] |
CHEN Hong-yan, ZHANG Tong-lai, ZHANG Jian-guo, et al. Synthesis, characterization and properties of tri-substitute potassium salt of trinitrophloroglucinol[J]. Chinese Journal of Chemistry, 2007, 25(1): 59. DOI:10.1002/(ISSN)1614-7065 |
| [3] |
CHEN Hong-yan, ZHANG Tong-lai, QIAO Xiao-jing, et al. Crystal structure and thermal behavior of rubidium 3, 5-dihydroxy-2, 4, 6-trinitrophenolate[J]. Chinese Journal of Inorganic Chemistry, 2006, 22(10): 1852. |
| [4] |
CHEN Hong-yan, ZHANG Tong-lai, ZHANG Jian-guo, et al. Crystal structure, thermal decomposition mechanism and explosive properties of[Na(H2TNPG)(H2O)2]n[J]. Journal of Hazardous Materials, 2006, 129(1-3): 31. DOI:10.1016/j.jhazmat.2005.08.014 |
| [5] |
CHEN Hong-yan, ZHANG Tong-lai, ZHANG Jian-guo, et al. The Preparation, structures and thermal decomposition mechanisms of trinitrophloroglucinol·hydrate[J]. Initiators & Pyrotechnics, 2005, 27(2): 13. |
| [6] |
CHEN Hong-yan, ZHANG Tong-lai, ZHANG Jian-guo, et al. Preparation, structure characterizations and thermal analysis of[Ba2(H2TNP)2(OH)2(H2O)2]·(CH3CH2OH)2·5H2O complex[J]. Chinese Journal of Energetic Materials(Hanneng Cailiao), 2006, 14(1): 21-26. |
| [7] |
CHEN Hong-yan, ZHANG Tong-lai, ZHANG Jian-guo. Preparation and molecular structure of (SCZ)(TNPG)·2H2O[J]. Chiniese Journal of Structural Chemistry, 2005, 24(8): 973-979. |
| [8] |
Sikder M N, Sikder A K, Survase D V, et al. Studies on 2, 4, 6-trinitrophloroglucinol (TNPG): A novel flash sensitizer[J]. Indian Journal of Engineering and Materials Sciences, 2004, 11(1): 59. |
| [9] |
Egorshev V Y, Sinditskii V P, Zbarsky V L. Synthesis and combustion study of metallic salts of trinitrophloroglucinol[J]. Theory and Practice of Energetic Materials, Parts A and B, 2004, 5: 30-37. |
| [10] |
CHEN Hong-yan, ZHANG Tong-lai, ZHANG Jian-guo, et al. Crystal structure, thermal decomposition and properties of cesium 3, 5-dihydroxy-2, 4, 6-trinitrophenolate[J]. Propellants, Explosives, Pyrotechnics, 2006, 31: 285. DOI:10.1002/(ISSN)1521-4087 |
| [11] |
Sinditskii V P, Levshenkov A I. The thermal decomposition of mono-, di-, and tripotassium salts of trinitrophloroglucinol[J]. Russian Journal of Physical Chemisty, 2009, 3(1): 46-55. DOI:10.1134/S1990793109010084 |
| [12] |
YANG Li, XUE Bing. Thermochemistry and thermodynamics of trinitrophloroglucinol dissolved in DMF and EtOH at 298.15[J]. Propellants, Explosives, Pyrotechnics, 2010, 33(5): 477-481. |
| [13] |
Marthada V K. The enthalpy of solution of SRM 1655(KCl) in H2O[J]. Journal of Research on NBS of Standards, 1980(85): 467-474. |
| [14] |
Klotz I M, Rosenberg I M. Chemical Thermodynamics[M]. Trans: BAO Yin-tang. Beijing: Peoples Education Press, 1982.
|
| [15] |
GAO Sheng-li, CHEN San-ping, HU Rong-zu. Derivation and application of thermodynamic equations[J]. Chinese Journal of Inorganic Chemistry, 2002(4): 362-366. |
Based on thermodynamics equation the experimental data are fitted by a linear least squares method, the kinetic formula of trinitrophloroglucinol (TNPG) dissolved in H2O is obtained. From the parameters of the kinetic formula, the rate constant of reaction (k) and the reaction order (n) can be calculated: n=0.78377, k=1.80×10-3 s-1.




